The Multifaceted Role of 2,2'-Dithiobis(benzothiazole) in Green Chemistry, Photochemical Dynamics, and Antitumor Activity
Feb 18,2024
General Description
The photochemical reaction dynamics of 2,2'-Dithiobis(benzothiazole) involve the rapid formation and decay of the benzothiazole-2-thiyl radical, studied through picosecond spectroscopy, revealing mechanisms of geminate recombination, dimerization, and a thiol–ene reaction with styrene. A green synthesis method via microwave irradiation significantly enhances its production, offering environmental benefits and efficiency. Furthermore, 2,2'-Dithiobis(benzothiazole) acts as a crucial ligand in synthesizing platinum and palladium complexes with notable antitumor and antibacterial activities, highlighting its potential in medical applications. These studies provide insights into its complex photochemical behaviors, efficient synthesis, and significant biological applications.

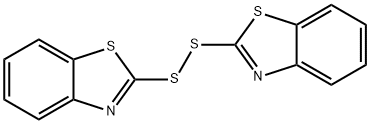
Figure 1. 2,2'-Dithiobis(benzothiazole)
Photochemical reaction dynamics
The photochemical reaction dynamics of 2,2'-Dithiobis(benzothiazole) involve the generation and subsequent reactions of the benzothiazole-2-thiyl (BS) radical upon ultraviolet photolysis at 330 nm. This process is scrutinized using picosecond transient electronic absorption spectroscopy (TEAS) and transient vibrational absorption spectroscopy (TVAS), revealing intricate details about the reaction mechanisms and kinetics on a picosecond time scale. Initially, the BS radical is formed almost instantaneously with a time constant of approximately 200 femtoseconds (fs). This rapid formation is followed by its decay through geminate recombination to 2,2'-Dithiobis(benzothiazole) and competitive dimerization, with a significant proportion surviving beyond 1.3 nanoseconds (ns) in methanol solution. A notable aspect of this study is the direct observation of a thiol–ene reaction between the BS radical and styrene, evidenced by the formation of the addition product, the BS–St radical. This reaction is characterized by distinct kinetic behaviors in different solvents. In styrene solution, an additional decay pathway for the BS radical is observed, with a time constant of 305 ps, indicating a bimolecular reaction with styrene. This interaction results in the formation of BS–St radicals, as confirmed by TVAS, which shows growth in specific absorption bands associated with the product. Furthermore, it is established that 22% of the initially formed BS radicals are converted into BS–St radicals in neat styrene solution. These findings underscore the complexity and efficiency of the photochemical reactions of 2,2'-Dithiobis(benzothiazole) providing valuable insights into the early stages of thiol–ene reactions, a pivotal class of chemical reactions, through the combined use of TEAS and TVAS with picosecond resolution. 1
Green Route for rapid synthesis
A study introduces a green and efficient method for synthesizing 2,2'-Dithiobis(benzothiazole) through microwave irradiation, significantly enhancing the synthesis process's speed and environmental friendliness. Traditionally, the conversion of thiols to disulfides, a key step in producing 2,2'-Dithiobis(benzothiazole), involves using H2O2 as an oxidizer and solvents like trifluoroethanol. However, when alternative solvents such as iso-propanol are used, the yield significantly drops, especially over extended reaction times (e.g., 24 hours). The novel approach discussed in this study leverages microwave irradiation to catalyze the synthesis of 2,2'-Dithiobis(benzothiazole) from its precursor molecule (M), drastically reducing the reaction time to merely 5 minutes. This method not only ensures a good yield of the desired product but also offers several advantages over traditional methods. These benefits include lower costs due to reduced energy and material requirements, easy purification processes, and notably, the elimination of harmful organic solvents, making this approach much more environmentally friendly. Overall, this green route for rapid synthesis of 2,2'-Dithiobis(benzothiazole) presents a significant advancement in the field of chemical synthesis, aligning with sustainability goals while maintaining efficiency and effectiveness. 2
Applications as a pivotal ligand
2,2'-Dithiobis(benzothiazole) serves as a pivotal ligand in the synthesis of novel mono- and binuclear platinum (Pt(II)) and palladium (Pd(II)) complexes, demonstrating significant applications in antitumor and antibacterial activities. The research introduces two complexes: [Pt(2,2'-Dithiobis(benzothiazole))(DMSO)Cl]Cl∙CHCl3 and [Pd2(µ-Cl)2(2,2'-Dithiobis(benzothiazole))2]Cl2, which have been synthesized and characterized through various analytical methods. These complexes exhibit distinct coordination modes with the metal ions interacting via N(3) atoms of the 2,2'-Dithiobis(benzothiazole) ligand. Notably, the Pt(II) complex shows dose- and time-dependent cytotoxicity against human breast cancer (MCF-7) and hepatocellular carcinoma (HepG2) cell lines, primarily through apoptosis and cell cycle arrest, while both complexes display antimicrobial effects against Escherichia coli and Kokuria rhizophila, with the Pt(II) complex being more potent. The differential intracellular uptake and mechanism of action between the complexes suggest a unique role of 2,2'-Dithiobis(benzothiazole) in modulating their biological activities, underscoring its potential in designing anticancer and antimicrobial agents. 3
Reference
1. Koyama D, Orr-Ewing AJ. Photochemical reaction dynamics of 2,2'-dithiobis(benzothiazole): direct observation of the addition product of an aromatic thiyl radical to an alkene with time-resolved vibrational and electronic absorption spectroscopy. Phys Chem Chem Phys. 2016;18(17):12115-12127.
2. Wang X, et al. Green Route for Rapid Synthesis of 2,2′-Dithiobis(benzothiazole) by Microwave Irradiation. Cheminform. 2009;39(19):3453-3458.
3. Rubino S, Busà R, Attanzio A, et al. Synthesis, properties, antitumor and antibacterial activity of new Pt(II) and Pd(II) complexes with 2,2'-dithiobis(benzothiazole) ligand. Bioorg Med Chem. 2017;25(8):2378-2386.
- Related articles
- Related Qustion
- 2,2'-Dithiobis(benzothiazole): properties, applications and toxicity Sep 25, 2023
2,2'-Dithiobis(benzothiazole) is a versatile compound with diverse applications including rubber industry and chemical synthesis.
- What is 2,2'-Dithiobis(benzothiazole)?Uses,Improve purities,Preparation Sep 9, 2020
2,2'-Dithiobis(benzothiazole) (DM) is an excellent accelerant for rubber vulcanization. It is widely used in rubber industry meanwhile it is a pharmaceutical intermediate with high activity.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDodecanedioic acid enhances metabolic health, enables sustainable industrial production, and supports regenerative medicine through innovative applications.....
Feb 18,2024API2,2'-Dithiobis(benzothiazole)
120-78-5You may like
2,2'-Dithiobis(benzothiazole) manufacturers
- 2,2'-Dithiobis(benzothiazole)
-

- $5.00 / 25KG
- 2025-11-19
- CAS:120-78-5
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 1000mt/year
- 2,2'-Dithiobis(benzothiazole)
-
- 2025-11-19
- CAS:120-78-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 2,2'-Dithiobis(benzothiazole)
-
- $5.00 / 1KG
- 2025-09-25
- CAS:120-78-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available