The Synthesis and Applications of Cannabidiol
Mar 5,2024
General Description
Cannabidiol is a naturally occurring compound derived from the Cannabis sativa plant. Unlike its well-known counterpart, tetrahydrocannabinol (THC), Cannabidiol is non-psychoactive, meaning it does not induce a "high" sensation commonly associated with cannabis use. Cannabidiol belongs to a class of chemical compounds known as cannabinoids, of which over a hundred have been identified in the cannabis plant. The endocannabinoid system, present in humans and many animals, interacts with these cannabinoids, playing a crucial role in regulating various physiological processes. One of the key distinctions of Cannabidiol is its therapeutic potential without the psychoactive effects. This characteristic has led to a surge in research exploring its applications across diverse fields, including health and wellness, skincare, and even sports and fitness. In the realm of healthcare, Cannabidiol has gained attention for its potential anti-inflammatory, analgesic, and anxiolytic properties.
Synthesis
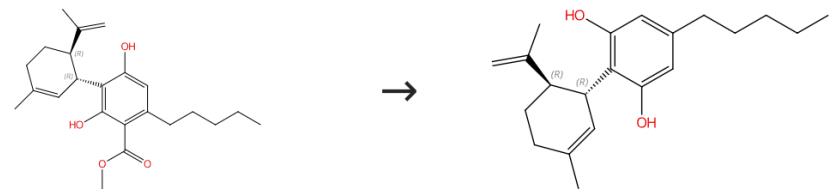
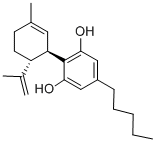
Fig. 1 The synthesis route of cannabidiol
Scheme 1:The toluene was removed by distillation and for the remaining first intermediate under stirring, 600 g of ethylene glycol was added and mixed with a solution of 85g of potassium hydroxide in 300 g of ethylene glycol. They placed a vacuum of about 0.5 bar and heated to 120°C for 2h, during which about 40 g of methanol was distilled. One the result of the transesterification, the product mixture comprised mainly cooled 2-Hydroxyethylcannabidiolate (III) at about 40 °C and added 500g of water and 500g of n-heptane and is to neutralize 150g half-conc. sulfuric acid was added. After phase separation, the solvent was spun off, and the residue on a thin film evaporator was distilled at a vacuum of about 0.5 mbar and a jacket temperature of 230°C. Yield: 60% of theory 310g. Purity: 85%. Viscous, yellowish oil. The synthesis route is shown in Fig. 1[1].
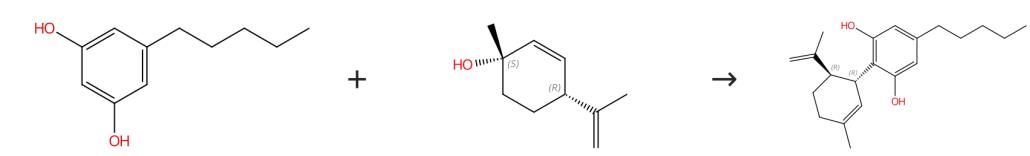
Fig. 2 The synthesis route of cannabidiol
Scheme 2:Synthesis of cannabidiol Olivetol (920 mg, 0.00511 mol) and p-toluene sulfonic acid (PTSA) ( 110 mg, 0.000578 mol) were dissolved in benzene; the resulting reaction mixture azeotrope for 2.5 h. The reaction mixture was cooled to room temperature and under argon (+)-(1S,4R)-p-Mentha- 2,8-dien-1-ol (586 mg, 0.00385 mol) was added and stirred at room temperature for 30 min. After completion of the reaction, the reaction mixture was diluted with diethyl ether and washed with saturated sodium bicarbonate solution. The organic layer separated and was dried on anhydrous sodium sulfate. The ether layer was evaporated and purified on a silica column by using petroleum ether and diethyl ether as eluent (98:2). Finally 300 mg of cannabidiol (300 mg) was collected (24%). 1H NMR (CDCl3): 6.23-6.41 (m,2H), 5.99 (s,1H,D2O exchangeable), 5.66 (s,1H), 4.56 (s,1H), 4.49 (bs,1H,D2O exchangeable) 4.46 (s,1H), 3.96 (m,1H), 2.00-2.47 (m,5H),1.87 (m,5H), 1.46-1.83 (m,6H), 1.23-1.48 (m,3H), 0.96-1.00 (m,3H).The synthesis route is shown in Fig. 1[2].
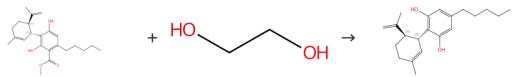
Fig. 3 The synthesis route of cannabidiol
Scheme 3:Transesterification, Synthesis of 2-Hydroxyethylcannabidiolat (III): The toluene was removed by distillation and 600 g of ethylene glycol was added under stirring for the remaining first intermediate and mixed with a solution of 85g of potassium hydroxide in 300 g of ethylene glycol. Place a vacuum of about 0.5 bar, and heated to 120°C for 2h, during which about 40 g of methanol was distilled. 2-hydroxyethyl cannabidiolate (III). Hydrolysis/decarboxylation, synthesis of cannabidiol (X): Thereafter, the temperature was raised to 150°C and stirred at this temperature for 2h. The product mixture resulting from the transesterification comprised mainly 2-Hydroxyethylcannabidiolate (III) at about 40°C and added with 500g water and 500g of n-heptane and is to neutralize 150g half-conc. sulfuric acid. After phase separation, the solvent is spun off, and the residue is distilled on a thin film evaporator at a vacuum of about 0.5 mbar and a jacket temperature of 230°C. Cannabidiol (X).
Applications
Cannabidiol has garnered significant attention in recent years due to its potential therapeutic benefits and diverse applications across various domains. Derived from the cannabis plant, Cannabidiol is one of over a hundred cannabinoids present in the plant, known for its non-psychoactive properties. One of the most well-known applications of Cannabidiol is in the field of healthcare and wellness. Studies have suggested that Cannabidiol exhibits anti-inflammatory, analgesic, and anxiolytic properties, making it a promising candidate for managing a range of conditions such as chronic pain, anxiety disorders, and even epilepsy. Epidiolex, a Cannabidiol-based medication, has been approved by the FDA for the treatment of certain types of epilepsy, marking a significant milestone in the recognition of Cannabidiol's therapeutic potential. Furthermore, Cannabidiol is increasingly being incorporated into skincare and beau.
References
[1]Mechoulam R, Parker L A, Gallily R. Cannabidiol: an overview of some pharmacological aspects[J]. The Journal of Clinical Pharmacology, 2002, 42(S1): 11S-19S.
[2]Pisanti, S., Malfitano, A. M., Ciaglia, E., Lamberti, A., Ranieri, R., Cuomo, G., ... & Bifulco, M. (2017). Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacology & therapeutics, 175, 133-150.
- Related articles
- Related Qustion
- Cannabidiol: pharmacology, clinical applications and toxicity Sep 26, 2023
Cannabidiol is a non-psychoactive compound from cannabis, effective in treating epilepsy, chronic pain, and anxiety.
- Introduction of CANNABIDIOL Aug 15, 2019
Cannabidiol is a chemical in the Cannabis sativa plant, also known as marijuana or hemp. Over 80 chemicals, known as cannabinoids, have been identified in the Cannabis sativa plant. While delta-9-tetrahydrocannabinol (THC) is the major active ingredient in marijuana, cannabidiol is also obtained from hemp, which contains only very small amounts of THC.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025API