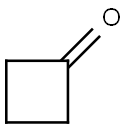
The Thermal Decomposition of Cyclobutanone
Jul 2,2025
Introduction
Cyclobutanone (Figure 1) is isosteric with the β‐lactam ring, with the quintessential β‐lactam nitrogen replaced by a carbon(Scheme 1). Since the β‐lactam nitrogen is effectively pyramidal by virtue of ring fusion in the penam and cephem ring systems, Gordon Lowe and others hypothesised that replacing this nitrogen with an sp3 hybridised carbon should “retain stereochemical compatibility with the active site of the transpeptidases and D,D-carboxypeptidases involved in bacterial cell wall biosynthesis.”Thus cyclobutanone analogues of β-lactams have been explored since the early 1980s, as potential antibiotics in their own right,as β-lactamase and serine protease inhibitors, and as mechanistic probes. It has been proposed that cyclobutanones-such as the generalised penam analogue 11-have the potential to inhibit serine β-lactamases via formation of an enzyme bound hemiketal with theactive site serine, and metallo β-lactamases via oxygen coordination to the metal ions.[1]As an important organic synthesis intermediate, further understanding of the thermal decomposition of cyclobutanone is needed.


Thermal Decomposition within the temperature range 333-373°
The homogeneous thermal decomposition of cyclobutanone has been investigated over the temperature range 333-373° The decomposition proceeds essentially by a reaction forming ethylene and ketene as the primary molecular products. For experiments conducted at initial pressures from 10 to 88 mm at 368° first-order kinetics were observed. The change in rate constant with temperature is given by the relationship ᶄ=3.6*1014 e-52000/RTsec.-1. The decomposition is not inhibited by the addition of propylene, toluene or nitric oxide. The thermal decomposition of cyclobutanone seems to be a simple reaction both from the viewpoint of the products formed and from the kinetics. The occurrence of essentially a single reaction (forming initially ethylene and ketene with only traces of other products) is in contrast to the photochemical results obtained at lower temperature. The failure of propylene, toluene or nitric oxide to decrease the rate of the reaction gives evidence that the decomposition is not a free radical chain process. The possibility that biradicals may be present momentarily as intermediates in the decomposition cannot be excluded. The trace of cyclopropane which has been reported in a mass spectrometric analysis might be taken as an indication that biradicals exist during the thermal decomposition as in the case of the photolytic reation. On the other hand, definite evidence for the presence of cyclopropane has not been obtained yet from the infrared observations. In view of the difficulty of analyzing for such a small amount of material and distinguishing it from traces of other substances, such as unremoved ketene or the pyrolytic products from ketene, it does not appear that a definite decision concerning the presence of cyclopropane can be made without further work.[2]
Induced by infrared radiation from a pulsed CO2 TEA laser
The decomposition of cyclobutanone vapor by infrared radiation from a pulsed CO2 TEA laser at 9.552 pm has been studied at pressures from about 0.1 to 10 Torr. Very minor amounts of propylene and acetylene were also observed. From the ratio of reaction [2] to reaction [1](Scheme 2), information was obtained about the effective temperature of the decomposition and the energy of the decomposing molecules. With the laser beam unfocussed, the decomposition at pressures above about 3 Torr is adequately described as a thermal decomposition, controlled by the initial temperature attained and the rate of cooling of the irradiated gas. At lower pressures, and when the laser beam was focussed, direct excitation and decomposition of individual molecules by multiphoton absorption appears to be involved.

It is useful to discuss the present results in terms of two limiting mechanisms. The first, which will predominate at high pressure and low fluence, can be described as a thermal decomposition of a hot gas approaching thermal equilibrium through collisional redistribution of energy. The second mechanism involves multiphoton absorption and dissociation of individual molecules essentially free of collisions,which can be expected to operate at low pressures and high fluence. Under conditions between these two extreme limits,some mixture of the two mechanisms will occur.The results with the unfocussed laser at pressures above about 3 Torr of cyclobutanone point clearly to a system tending towards the first mechanism, a thermal decomposition, rather than a multiphoton dissociation. At the highest fluence employed, an average of about 4 photons per molecule were absorbed during the ~250 ns pulse, while at 3 Torr pressure each molecule would suffer on the average about 25 collisions during the same period.Collisional energy transfer would obviously compete effectively with excitation, and the direct accumultion in a single molecule of the 17 quanta necessary for dissociation would become very improbable. At lower fluence and higher pressure collisional processes would become even more dominant. To test the plausibility of a thermal decomposition model under such conditions, a simple calculation can be made of the maximum temperature to be expected in the irradiated gas. At a pressure of 9.5 Torr the average total energy absorbed in a single pulse at the highest fluence was 17.9 kcal/mol. The heat capacity of cyclobutanone was calculated by the group additivity method outlined by Benson and O'Neal (10) at intervals of 100°, and a graphical integration performed to estimate a temperature of 880 K at the end of the pulse, assuming thermal equilibration in the irradiated zone and negligible cooling.[3]
Conclusion
(1)Multiple photon dissociation of cyclobutanone with 9.6 μm R(14) TEA CO2 laser line proceeds along the same two channels as those observed in the thermal decomposition;the major one yields ethylene and ketene, and the minor one,cyclopropane and CO. Under stronger irradiations, the primary produced cyclopropane partly isomerizes to propylene, and some subsequent multiple photon absorptions of the primary products result in the formation of secondary products,particularly acetylene.
(2)From the comparison between the experimental results and a statistical model calculation concerning the laser energy effect on the product yields, the branching ratio, and the degrees of the subsequent isomerization from the primary produced cyclopropane, no sound evidence is found to reject the hypothesis that, during the laser pulse,the cyclobutanone molecules remain in external and also internal equilibria and dissociates at rates which are predicted from the unimolecular rates observed in conventional thermal experiments.[4]
References
[1]Devi P, Rutledge PJ. Cyclobutanone Analogues of β-Lactam Antibiotics: β-Lactamase Inhibitors with Untapped Potential?. Chembiochem. 2017;18(4):338-351. doi:10.1002/cbic.201600529
[2] Das MN , Kern F , Coyle TD ,etal.The Thermal Decomposition of Cyclobutanone1[J].Journal of the American Chemical Society, 1954, 76(24):6271-6274.DOI:10.1021/ja01653a013.
[3] Back M H , Back R A .The decomposition of cyclobutanone vapor induced by infrared radiation from a pulsed CO 2 TEA laser[J].Canadian Journal of Chemistry, 1979.DOI:10.1139/v79-247.
[4] Koda S , Ohnuma Y , Ohkawa T ,et al.Infrared multiple photon dissociation of cyclobutanone[J].Bulletin of the Chemical Society of Japan, 1980, 53(12):3447-3456.DOI:10.1246/bcsj.53.3447.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPhenyltrimethoxysilane is a widely used organic siloxane in industry. Herein, this report will introduce its application research.....
Jul 3,2025Organic Synthesis IntermediateCyclobutanone
1191-95-3You may like
- γ-Valerolactone:synthesis and chemical recovery
Dec 15, 2025
- Application research of 4,4'-Dibromobiphenyl
Nov 18, 2025
- Application research of Octadecyl acrylate
Nov 18, 2025
- Cyclobutanone
-
- $0.00 / 1kg
- 2025-07-08
- CAS:1191-95-3
- Min. Order: 0.10000000149011612kg
- Purity: 99.0%min
- Supply Ability: 200kg
- Cyclobutanone
-

- $5.00 / 1KG
- 2025-05-26
- CAS:1191-95-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg
- Cyclobutanone
-

- $0.00 / 1kg
- 2024-02-26
- CAS:1191-95-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Kgs