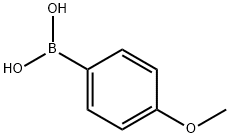
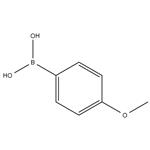
The uses of 4-Methoxyphenylboronic acid
Jan 2,2020
4-Methoxyphenylboronic acid is a reagent used in the preparation of various biological inhibitors.
Uses
4-Methoxyphenylboronic acid is a reagent used for Suzuki-Miyaura cross-coupling reactions1;Pd-catalyzed direct arylation;Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water;Palladium-catalyzed stereoselective Heck-type reaction;Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence;Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides;Ruthenium catalyzed direct arylation;Rh-catalyzed asymmetric conjugate addition. Studies on 4-methoxyphenylboronic acid is a thioformamide palladium complex as a Suzuki coupling catalyst. 4-methoxyphenylboronic acid push-pull arylvinyldiazine chromophore with photophysical properties.
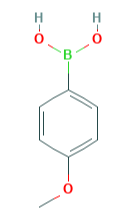
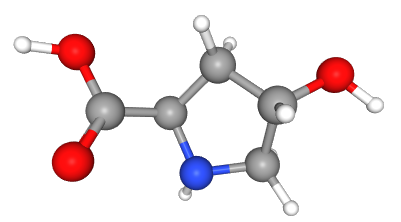
Fig 1. Chemical structure formula and three-dimensional structure of 4-Methoxyphenylboronic acid
4-Methoxybenzeneboronic acid is used for Suzuki-Miyaura cross-coupling reactions, Pd-catalyzed direct arylation, Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, Palladium-catalyzed stereoselective Heck-type reaction, Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, Ruthenium catalyzed direct arylation, Rh-catalyzed asymmetric conjugate addition, Ligand-free copper-catalyzed coupling[1-3].
Improved syntheses of optically pure(-)- and (+)- morinol C, and (-)- and (+)-morinol D were achieved by employing the Mizoroki-Heck reaction to construct the cinnamyl moiety. The protective group of the alkene substrate affected the yield of this key reaction. The reaction with a combination of the acetate-protected olefin and 4-methoxyphenylboronic acid gave the best result producing morinol C and D.All stereoisomers of morinol C and D showed cytotoxic activity, with (R,R)-morinol C showing the highest antibacterial activity[4].
Simultaneous chemical vapor deposition (CVD) of graphene and “in-situ” phosphorous or boron doping of graphene was accomplished using Triphenylphosphine (TPP) and 4-Methoxyphenylboronic acid. The TPP and 4-MPBA molecules were sublimated and supplied along with CH4 molecules during graphene growth at atmospheric pressure. The grown graphene samples were characterized using Raman spectroscopy. Phosphorous and boron presence in phosphorous and boron doped graphene was confirmed with Auger electron spectroscopy. The possibility of obtaining phosphorous and boron doped graphene using solid-source molecule precursors via CVD can lead to an easy and rapid production of modified large area graphene[5].
Supramolecular assemblies of phenylboronic and 4-methoxyphenylboronic acids with 4,40-bipyridine and an assembly of phenylboronic acid with 1,2-bis(4-pyridyl)ethene, which were obtained due to the formation of O–H...N hydrogen bonds between hetero N-atoms and –B(OH)2 are reported. Further, a centrosymmetric cyclic C–H...O hydrogen bonding dimer is identified in the crystal structure of 4-methoxyphenylboronic acid[6].
References
1.S.A. Barker.; A.K. Chopra.; B.W. Hatt.; P.J. Somers. The interaction of areneboronic acids with monosaccharides . Carbohydrate Research. 1973, 26 (1), 33-40.
2.S.A. Barker, B.W. Hatt, P.J. Somers. The effect of areneboronic acids on the alkaline conversion of d-glucose into d-fructose. Carbohydrate Research . 1973, 26 (1), 41-53.
3.For an illustrative example of the silver oxide promoted Suzuki coupling with sensitive ɑ-halo enones, under extremely mild conditions, see: Org. Synth., 75, 69 (1997).
4.OGURA, Koji, SUGAHARA, Takuya, MARUYAMA, Masafumi. Improved Syntheses of Morinol C and D by Employing Mizoroki-Heck Reaction and Their Cytotoxic and Antimicrobial Activities[J]. Biosci Biotechnol Biochem, 74(8):1641-1644.
5.Ovezmyradov M , Magedov I V , Frolova L V , et al. Chemical Vapor Deposition of Phosphorous- and Boron-Doped Graphene Using Phenyl-Containing Molecules[J]. Journal of Nanoscience and Nanotechnology, 2015, 15(7):4883-4886.
6.Zotto A D , Amoroso F , Baratta W , et al. Very Fast Suzuki-Miyaura Reaction Catalyzed by Pd(OAc)2 under Aerobic Conditions at Room Temperature in EGME/H2O[J]. European Journal of Organic Chemistry, 2009, 2009(1):7.
- Related articles
- Related Qustion
- The application of 4-methoxyphenylboronic acid in organic synthesis Sep 29, 2025
4-Methoxyphenylboronic acid can be used as a organic reagent in Suzuki coupling reaction. This paper mainly reports the its application in organic synthesis
- 4-Methoxyphenylboronic acid: applications in different fields and safety Oct 11, 2023
4-Methoxyphenylboronic acid is a versatile compound with applications in electrochemical sensing, graphene synthesis, and organic reactions.
Tert-butyl acetoacetate(t -BAA) is a colorless liquid, stable under normal temperature and pressure, avoiding strong oxidant contact.....
Jan 2,2020Chemical Reagents1,1'-Thiocarbonyldiimidazole (TCDI) is a thiourea containing two imidazole rings.It is the sulfur analog of the peptide coupling reagent carbonyldiimidazole (CDI).....
Jan 3,2020Organic Chemistry4-Methoxyphenylboronic acid
5720-07-0You may like
4-Methoxyphenylboronic acid manufacturers
- 4-Methoxyphenylboronic acid
-

- $0.00 / 25kg
- 2025-12-01
- CAS:5720-07-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- 4-Methoxyphenylboronic acid
-
- $34.00 / 1kg
- 2025-09-25
- CAS:5720-09-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
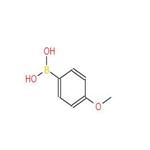
- 4-Methoxyphenylboronic acid
-
- $30.00/ Kg
- 2022-09-28
- CAS:5720-09-0
- Min. Order: 1Kg
- Purity: 99.0% up
- Supply Ability: 50 tons per month