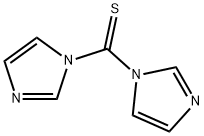
1,1'-Thiocarbonyldiimidazole-Application
Jan 3,2020
1,1'-Thiocarbonyldiimidazole (TCDI) is a thiourea containing two imidazole rings.It is the sulfur analog of the peptide coupling reagent carbonyldiimidazole (CDI).
Trans-4-hydroxy-D-proline is a 4-hydroxy-D-proline in which the hydroxy group at position has S-configuration.1,1'-Thiocarbonyldiimidazole is commercially available but can also be prepared via the reaction of thiophosgene with two equivalents of imidazole.The imidazole groups on 1,1'-Thiocarbonyldiimidazole can be easily displaced, allowing it to act as a safer alternative to thiophosgene. This behaviour has been used in the Corey–Winter olefin synthesis. It may also replace carbonothioyl species (RC(S)Cl) in the Barton–McCombie deoxygenation. Other uses include the synthesis of thioamides and thiocarbamates. Like the analogous CDI, it may be used for peptide coupling[1-3].
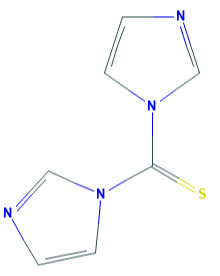

Fig 1. Chemical structure formula and three-dimensional structure of 1,1'-Thiocarbonyldiimidazole
The efficient and simple routes for the synthesis of various ferrocenyl derivatives from ferrocenylcarbinols and N,N’-thiocarbonyldiimidazole are described. It involves grinding the two substrates in a Pyrex tube with a glass rod at room temperature. The reaction of ferrocenylmethanol provided S,S-bis(ferrocenylmethyl)dithiocarbonate, whose crystal structure and a plausible mechanism for its formation are also reported. The reaction of 1-ferrocenyl-1-phenylmethanol and 1-ferrocenylbutanol gave the products 2c and 2d, respectively. The reaction of ω-ferrocenyl alcohols 4-ferrocenylphenol and 6-ferrocenylhexan-1-ol yielded the products 3c and 3d, respectively. Reaction of 1,1′-ferrocenedimethanol afforded 3f in moderate yield, and by contrast, it was not similar to 1b. Reaction of [4-(trifluoromethyl)phenyl]methanol provided the thiocarbonate in good yield.
1,1'-Thiocarbonyldiimidazole is used as a reagent for the deoxygenation of alcohols with tributyltin hydride and diols with lithium aluminum hydride. It is also used to deoxygenate carboxylic monosaccharide analogues. Further, it is a useful reagent involved in biochemical synthesis. In addition to this, it is used to prepare 1-[bis-(4-methoxy-phenyl)-methyl]-1H-imidazole by reacting with 4,4'-dimethoxy-benzhydrol[4-6].
The Corey-Winter alkene synthesis is an effective method for the deoxygenation of vicinal diols.The method involves formation of a 1,3-dioxolane-2-thione [cyclic thionocarbonate (or thiocarbonate)] by treatment of a vicinal diol with 1,1'-Thiocarbonyldiimidazole. Decomposition of the thionocarbonate, usually with a phosphorus compound, affords the alkene. The breakdown of the thionocarbonate occurs in a stereospecific sense; details of investigations into the mechanism have been summarized[7].
Treatment of secondary alcohols with 1 equiv of 1,1'-Thiocarbonyldiimidazole affords an imidazole-1-thiocarbonyl derivative (imidazolide), which can be reduced to a CH2 unit under Tri-n-butylstannane (TBTH) radical chain reaction conditions. The deoxygenation of secondary alcohols by way of an imidazolide or other thiocarbonyl derivative is called the Barton-McCombie reaction. Since imidazolide formation (1,1'-Thiocarbonyldiimidazole, reflux, 65℃) and the subsequent radical chemistry are done under neutral or near-neutral conditions, the overall reduction is tolerant of the presence of many sorts of functional groups. Furthermore, the low solvation requirements of radical species permits deoxygenation in sterically congested environments[8].
References
1.Adrian, L. Schwan; Jeffrey, H. Byers (15 March 2007). "1,1′‐Thiocarbonyldiimidazole". Encyclopedia of Reagents for Organic Synthesis.
Corey, E. J.; Winter, Roland A. E. (September 1963). "A New, Stereospecific Olefin Synthesis from 1,2-Diols". Journal of the American Chemical Society. 85 (17): 2677–2678.
2.Esser, Franz; Roos, Otto (June 1978). "N-Terminal Cyclization of Peptides withN,N′-Carbonyldiimidazole orN,N′-Thiocarbonyldiimidazole". Angewandte Chemie International Edition in English. 17 (6): 467–468.
3.Reacts with diols to give cyclic thionocarbonates which are desulfurized by trialkyl phosphites to give alkenes by cis-elimination of CO2 from the intermediate carbene (Corey-Winter olefination): J. Am. Chem. Soc., 85, 2677 (1963); Angew. Chem. Int. Ed., 14, 753 (1975).
4.For a modified procedure involving ring-opening of the thionocarbonate intermediate with an alkyl iodide, followed by Zn reduction of the resulting iodo ester, see: Tetrahedron Lett., 3793 (1973).
5.Ivanov, A. V.; Virus, E. D.; Luzyanin, B. P.; Kubatiev, A. A. Capillary electrophoresis coupled with 1,1'-thiocarbonyldiimidazole derivatization for the rapid detection of total homocysteine and cysteine in human plasma. J. Chromatogr. B 2015, 1004, 30-36.
6.Corey, E. J.; and Winter, R. A. E. JACS 1963, 85, 2677.
7.Manchand, P. S.; Belica, P. S.; Holman, M. J.; Huang, T.-N.; Maehr, H.; Tam, S. Y.-K.; Yang, R. T. JOC 1992, 57, 3473.
- Related articles
- Related Qustion
- 1,1'-Thiocarbonyldiimidazole: properties, applications and safety Dec 8, 2023
1,1'-Thiocarbonyldiimidazole is a versatile and reactive compound used in organic synthesis and analytical chemistry with strict safety protocols.
4-Methoxyphenylboronic acid is a reagent used in the preparation of various biological inhibitors.....
Jan 2,2020Chemical Reagents3,4-Dimethoxybenzaldehyde, also known as methyl vanillin, methyl vanillin, , having a vanilla (Vanillabeans ) fragrances synthetic perfumes, but also uses more pharmaceutical intermediates.....
Jan 6,2020Natural Products1,1'-Thiocarbonyldiimidazole
6160-65-2You may like
1,1'-Thiocarbonyldiimidazole manufacturers
- 1,1'-Thiocarbonyldiimidazole
-

- $0.00 / 1KG
- 2025-06-27
- CAS:6160-65-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Thiocarbonyldiimidazole
-

- $2.00 / 1kg
- 2025-06-09
- CAS:6160-65-2
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 10000kg
- 1,1'-Thiocarbonyldiimidazole
-

- $0.00 / 1KG
- 2025-04-04
- CAS:6160-65-2
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton