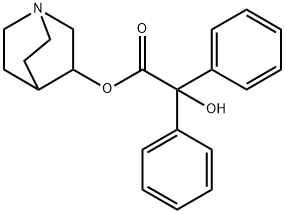
Toxicity of 3-Quinuclidinyl benzilate
Dec 3,2021
3-Quinuclidinyl benzilate (QNB) — IUPAC name 1-azabicyclo[2.2.2]octan-3-yl hydroxy(diphenyl)acetate; US Army code EA-2277; NATO code BZ; Soviet code Substance 78— is an odorless and bitter-tasting military incapacitating agent.BZ is an antagonist of muscarinic acetylcholine receptors whose structure is the ester of benzilic acid with an alcohol derived from quinuclidine.
Physiochemical characteristics
BZ is odorless. It is a white crystalline powder with a bitter taste. It is stable in most solvents, with a half-life of three to four weeks in moist air; even heat-producing munitions can disperse it. It is extremely persistent in soil and water and on most surfaces. BZ is soluble in water, soluble in dilute acids, trichloroethylene, dimethylformamide, and most organic solvents, and insoluble with aqueous alkali.
Uses
The 3-quinuclidinyl benzilate (3-QNB) was first discovered by Hoffmann-La Roche in 1951 during research investigation that involved development of antispasmodic agents, resembling atropine, for treating gastrointestinal conditions. Soon after, the US Army investigated 3-QNB under the name of EA2277 agent and was standardized for use in chemical munitions in 1961 under the name agent of BZ. BZ is a nonlethal chemical warfare agent that following a delayed onset is incapacitating and causes severe hallucinations. US Army intended to use BZ in critical point situations such as special operation, incapacitating adversaries during raid, and overcoming fortified field positions. Medically, BZ is used as a muscarinic receptor antagonist.
Effects
As a powerful anticholinergic agent, BZ produces a syndrome of effects known as the anticholinergic toxidrome: these include both psychological and physiological effects, with the most incapacitating effect being a state of delirium characterized by cognitive dysfunction, hallucinations, and inability to perform basic tasks. The usual syndrome of physical anticholinergic effects are also present, including mydriasis (potentially to the point of temporary blindness), tachycardia, dermal vasodilation, xerostomia and hyperthermia.The readily-observable symptoms of the anticholinergic toxidrome are famously characterized by the mnemonic "Mad as a hatter, red as a beet, dry as a bone and blind as a bat" (and variations thereof).
Toxicity
Based on data from more than 500 reported cases of accidental atropine overdose and deliberate poisoning, the median lethal oral dose is estimated to be approximately 450 mg (with a shallow probit slope of 1.8). Some estimates of lethality with BZ have been grossly erroneous, and ultimately the safety margin for BZ is inconclusive due to lack of human data at higher dosage ranges. Though some researchers have estimated it to be 0.5 to 3.0 mg/kg and an LD01 is 0.2 to 1.4 mg/kg (Rosenblatt, Dacre, Shiotsuka, & Rowlett, 1977).
- Related articles
- Related Qustion
Butyric acid is a four-carbon acid with an unpleasant and obnoxious odor, with a butter-fat taste. It is a clear and colorless oily liquid and occurs in butter and animal fat as the glycerol ester. n-Butyric acid is manufactured in enclosed....
Dec 3,2021Organic ChemistryDiscovered in 1817 as an impurity in calamine (zinc carbonate) by Friedrich Stromeyer and Karl Samuel Leberecht Hermann while working independently, cadmium exists in abundance of w0.1 ppm in the Earth’s crust.....
Dec 3,2021Inorganic chemistry