Toxicity of Garenoxacin
Mar 18,2022
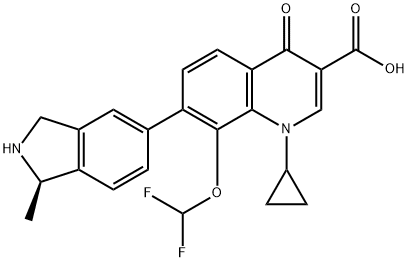
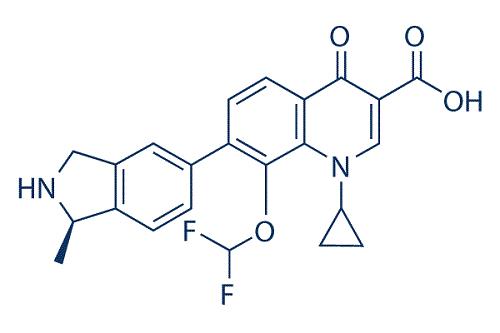
Garenoxacin is a desfluoro quinolone which, as such, lacks a fluorine at the C-6 position in comparison with other fluoroquinolones, such as ciprofloxacin, moxifloxacin, and levofloxacin, and has a difluoromethoxy substitution at position C-8. The chemical structure is 1-cyclopropyl-8-(difluoromethoxy)-7-[(1R)- (1-methyl-2, 3-dihydro-1H-5-isoindolyl)-4-oxo-1, 4-dihydro-3-quinolinecarboxylic acid] methanesulfonate monohydrate.
Like other members of this class, garenoxacin has activity against many Gram-positive and Gram-negative bacteria and intracellular respiratory pathogens. In particular it has activity against penicillinsusceptible and -resistant Streptococcus pneumoniae, methicillin-susceptible Staphylococcus aureus (MSSA) and some anaerobes.
Mechanism of action
The quinolone class of antibacterials act by inhibition of bacterial topoisomerases. During DNA replication DNA gyrase (a tetramer composed of four subunits, two GyrA and two GyrB) catalyses ATPdependent DNA supercoiling. In the contact region of the tetramer, double-stranded DNA is cleaved (the G-segment), while a second region of DNA (the T-segment) is captured and passed through the cleaved DNA gate using ATP as a source of energy. The G-segment is then rejoined and the T-segment exits the tetramer. The DNA gyrase can remain attached to the G-segment with repeated capture of T-segments after each round of ATP hydrolysis. Like DNA gyrase, topoisomerase IV is a tetramer consisting of four subunits, two each of ParC and ParE, that can remove supercoils. Unlike DNA gyrase, however, topoisomerase IV can also remove precatenanes that are formed behind the replication fork.
Fluoroquinolones act by the formation of drug–enzyme–DNA complexes that trap the gyrase and topoisomerase IV on the DNA resulting in inhibition of DNA synthesis. A second process, fragmentation of the chromosome, results in rapid cell death. Garenoxacin inhibits both DNA gyrase (topoisomerase II) and topoisomerase IV.
Toxicity
Neurotoxicity
Dizziness, headaches, and somnolence are the most frequently reported central nervous system (CNS) side-effect after quinolones administration with an overall incidence of 1–2%. More severe adverse events, such as agitation, delirium, psychosis, and seizures, have also been reported.
The incidence of these side-effects varies substantially depending on the quinolone used. This is thought to be related to the capacity of the quinolone to displace gamma-aminobutyric acid from its receptor in the CNS, resulting in a general stimulating effect. This effect seems to be potentiated by the concomitant administration of anti-inflammatory drugs, for example fenbufen. Because of its different chemical structure, the CNS effects of garenoxacin were weaker than those of other quinolones in a mouse model. CNS toxicity events reported in the phase II/III trials were headaches, dizziness, insomnia, anxiety, confusion, and agitation. However, their incidence was similar to the comparator (17.5% vs 19.7%, respectively).
- Related articles
- Related Qustion
Gatifloxacin is an 8-methoxy fluoroquinolone and is marketed by Bristol Myers Squibb as Tequint. Three formulations are available: oral, ophthalmic, and intravenous. Like other members of this class, gatifloxacin has activity against many G....
Mar 18,2022APIGlyphosate is a biocide herbicide developed by the famous American company Monsanto.....
Mar 21,2022APIGarenoxacin
194804-75-6You may like
- Rolapitant Synthesis
Dec 22, 2025
- Synthesis of 2-(2-Chlorophenyl)cyclohexanone
Dec 22, 2025
- Preparation methods and application of 2-(2-Ethoxyethoxy)ethyl acrylate
Dec 22, 2025
- Garenoxacin
-

- $0.00 / 50G
- 2025-12-05
- CAS:194804-75-6
- Min. Order: 1G
- Purity: 99.99%
- Supply Ability: 1KGS
- Garenoxacin USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:194804-75-6
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- Garenoxacin
-

- $0.00 / 25kg
- 2025-05-12
- CAS:194804-75-6
- Min. Order: 1kg
- Purity: 98% HPLC
- Supply Ability: 500 kgs