Trimethoxysilane: Toxicology & In Situ Hydrolysis Monitoring
Dec 11,2025
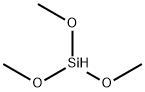
Trimethoxysilane (TMS) is an organosilicon compound with the formula HSi(OCH3)3. The compound is a commonly used basic raw material for the preparation of silicone materials. Trimethoxysilane is an important substance for producing silane coupling agents. It contains both hydrolyzable siloxane bonds as well as an active silicon-hydrogen bond.

Toxicology of Trimethoxysilane
Trimethoxysilane and tetramethoxysilane are organosilanes (silicon esters). Silicon esters are silicon compounds that contain an oxygen bridge from silicon to an organic group (Si-OR). These compounds are classified according to whether the Si-OR bond is expected to remain intact or hydrolyzed in the final application. Trimethoxysilane and tetramethoxysilane are both alkoxysilanes and generally have sweet-fruity odors that become less apparent as the molecular weight increases. Both chemicals can be absorbed into the corneal tissue and cause eye damage (Arkles 2000). Tetramethoxysilane is used in the ceramic industry for closing pores, for coating metal surfaces, and as a bonding agent in paints and lacquers. Trimethoxysilane is used as an intermediate for producing silane. Whereas inorganic silanes are oxidized spontaneously on contact with oxygen and air, the organosilanes are more stable. The 1-h LC50 (with 95% confidence level) was 161 ppm for males, 146 ppm for females, and 154 ppm for both sexes. Those values were determined by a modified method of Finney (1964) using probit analysis. Mortalities were observed at all of the concentrations except 19 ppm in the 4-h group and 68 ppm in the 1-h group, with all deaths occurring during the 14-day post-exposure observation period.[1]
Trimethoxysilane and tetramethoxysilane are colorless liquids with ester-like odors. They are structural analogs and are in the organic silane family. Both chemicals have similar toxicologic effects in the lung and eye. Little relevant data on the toxicity of the chemicals in either humans or laboratory animals are available.AEGL-1 values were not recommended for trimethoxysilane because of inadequate data. Data were also inadequate to derive AEGL-2 values, so values were derived by taking one-third of the AEGL-3 values. AEGL-3 values for trimethoxysilane were determined on the basis of mortality data from 1- and 4-h LC50 (lethal concentration, 50% lethality) inhalation studies in rats. Points of departure were the calculated LC01 (lethal concentration, 1% lethality) values of 263 ppm for 10 min, 123 ppm for 30 min, 76.3 ppm for 1 h, 29.3 ppm for 4 h, and 18.2 ppm for 8 h. A total uncertainty factor of 30 was used. A factor of 3 was applied for interspecies differences, because similar effects were observed in rats, mice, and hamsters exposed at the same concentration in a 5-day inhalation study. The default value of 10 was used for intraspecies variability, because no data were available to estimate human variability and it was not clear that trimethoxysilane acts as a simple chemical irritant in the lungs. Time scaling was performed using the concentration-time relationship equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 0.8 to 3.5. An empirical value for n of 1.45 was calculated for trimethoxysilane.
Simultaneous In Situ Monitoring of Trimethoxysilane Hydrolysis Reactions
Sol-gels are found in many different scientific fields and have very broad applications. They are often prepared by the hydrolysis and condensation of alkoxysilanes such as trimethoxysilanes, which are commonly used as precursors in the preparation of silsequioxanes via the sol-gel process. The reaction rates of such reactions are influenced by a wide range of experimental factors such as temperature, pH, catalyst, etc. In this study, we combined multiple in situ spectroscopic techniques to monitor the hydrolysis and partial condensation reactions of methyltrimethoxysilane and phenyltrimethoxysilane. A rich set of kinetics information on intermediate species of the hydrolysis reactions were obtained and used for kinetics modeling. Raman and nuclear magnetic resonance (NMR) spectroscopy provided the most information about hydrolysis and NMR provided the most information about condensation. A quantitative method based on Raman spectra to quantify the various transient intermediate hydrolysis products was developed using NMR as the primary method, which can be deployed in the field where it is impractical to carry out NMR measurements.[2]
References
[1]Committee on Acute Exposure Guideline Levels; Committee on Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 13. Washington (DC): National Academies Press (US); 2012 Dec 28. 7, Trimethoxysilane and Tetramethoxysilane.
[2]Chen, Xiaoyun et al. “Simultaneous In Situ Monitoring of Trimethoxysilane Hydrolysis Reactions Using Raman, Infrared, and Nuclear Magnetic Resonance (NMR) Spectroscopy Aided by Chemometrics and Ab Initio Calculations.” Applied spectroscopy vol. 72,9 (2018): 1404-1415. doi:10.1177/0003702818774570
- Related articles
- Related Qustion
Mavacamten is a small-molecule allosteric and reversible inhibitor of cardiac myosin ATPase. Herein its relevant information were introduced.....
Dec 11,2025DrugsThe basic structure of methyl 2-benzoylbenzoate is similar to di(2-ethylhexyl) phthalate. Herein the effects of photoinitiators were introduced.....
Dec 11,2025Chemical MaterialsTrimethoxysilane
2487-90-3You may like
Trimethoxysilane manufacturers
- Trimethoxysilane
-
- 2025-12-11
- CAS:2487-90-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Trimethoxysilane
-

- $10.00 / 1KG
- 2025-12-11
- CAS:2487-90-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Trimethoxysilane
-

- $0.00 / 25kg
- 2025-12-01
- CAS:2487-90-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS