Unveiling the Chemistry of 2-Methylbutane: A Comprehensive Insight into Synthesis, Composition, Applications, and Storage
May 13,2024
Introduce
2-Methylbutane, commonly known as isopentane, stands as a cornerstone in the realm of organic chemistry, capturing the interest and curiosity of professionals worldwide. Its intricate synthesis pathways, nuanced properties, and diverse applications serve as focal points of exploration and study within the scientific community. The distinct molecular structure of 2-methylbutane, characterized by its branched-chain configuration and methyl group, not only contributes to its unique chemical behavior but also underpins its manifold utility across various industries. Chemists and researchers, drawn by its versatility and significance, continually seek to unravel the intricacies of 2-methylbutane, probing its synthesis mechanisms, unraveling its chemical interactions, and uncovering novel applications. As such, the journey to comprehend the full spectrum of 2-methylbutane's capabilities remains an ongoing endeavor, driving scientific inquiry and innovation in the field of organic chemistry[1].

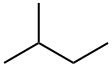
Figure 1 Characteristics of 2-Methylbutane
Synthesis method
The synthesis of 2-methylbutane is primarily achieved through the alkylation process, wherein isobutane reacts with methanol in the presence of acidic catalysts, typically zeolites. This reaction initiates the formation of isobutylene, which subsequently undergoes further reaction with methanol to yield 2-methylbutane. Additionally, an alternative method involves the catalytic dehydrogenation of 2-methylbutane, facilitated by transition metal catalysts, providing an alternative route for its production.
Main ingredients
With a molecular formula of C5H12, 2-methylbutane is characterized as a branched-chain alkane. Its molecular structure comprises a central carbon atom bonded to three other carbon atoms and a methyl group, rendering it a colorless, volatile liquid at ambient temperature.
Application
The versatility of 2-methylbutane extends beyond conventional applications, permeating diverse industries and niches with its multifaceted properties. In the pharmaceutical sector, it serves as a crucial solvent in the formulation of medications, facilitating the dissolution of active pharmaceutical ingredients and aiding in the production of pharmaceutical formulations. Its compatibility with various organic compounds makes it indispensable in the synthesis of pharmaceutical intermediates and active pharmaceutical ingredients (APIs), contributing to the development of novel therapeutic agents.
Moreover, 2-methylbutane finds utilization in the realm of analytical chemistry, where its inert nature and volatility make it an ideal choice for gas chromatography applications. As a carrier gas or mobile phase, it enables the separation and analysis of complex mixtures with high precision and sensitivity, facilitating qualitative and quantitative analysis in research laboratories and quality control settings.
Furthermore, the unique properties of 2-methylbutane render it invaluable in the field of biotechnology, particularly in cryopreservation techniques. Its low boiling point and rapid evaporation rate make it well-suited for flash-freezing biological samples, such as cells, tissues, and embryos, preserving their viability and integrity for long-term storage and experimental manipulation.
Additionally, 2-methylbutane finds niche applications in the aerospace industry, where its combination of low boiling point and high energy content makes it an attractive fuel for certain propulsion systems and rocket engines. Its use as a propellant in aerosol formulations further underscores its versatility and adaptability across disparate sectors, reaffirming its status as a versatile compound with boundless potential.
Storage method
Given its flammable nature, the proper storage of 2-methylbutane is paramount to mitigating potential hazards. It should be stored in tightly sealed containers placed in well-ventilated areas, away from potential ignition sources such as heat, sparks, and open flames. Additionally, adherence to established safety protocols and regulations is imperative to ensure the safe handling and storage of this compound.
Moreover, regular inspection and maintenance of storage facilities are essential to uphold safety standards and prevent potential leaks or accidents. Proper labeling and signage should also be employed to communicate the hazards associated with 2-Methylbutane and guide personnel in its safe handling and storage procedures.
Conclusion
In summation, 2-methylbutane emerges as a multifaceted compound with significant implications across numerous domains, including organic synthesis, cryogenics, and fuel technology. By elucidating its synthesis, composition, applications, and storage requirements, this article aims to equip professionals in the chemistry field with the requisite knowledge to leverage the capabilities of 2-methylbutane effectively while prioritizing safety and efficacy in its utilization[2].
References
[1]Galvin J B, Marashi F. 2-Methylbutane (isopentane)[J]. Journal of Toxicology and Environmental Health Part A, 1999, 58(1-2): 23-33.
[2]Hafezi M J, Sharif F. Study of the torsional potential energies of 2-methylpropane, n-butane, and 2-methylbutane with high-level ab initio calculations[J]. Journal of Molecular Structure: THEOCHEM, 2007, 814(1-3): 43-49.
- Related articles
- Related Qustion
- 2-Methylbutane: Properties, Uses, and Safety Considerations in Industrial Applications Jan 26, 2024
2-Methylbutane, a low-molecular-weight alkane derived from natural gas and crude oil, is versatile and safe for use as a solvent, building-block, and propellant in industrial processes and cosmetics.
- 2-Methylbutane: toxicology, pharmacokinetics and applications Jul 19, 2023
2-Methylbutane can irritate the skin, cause respiratory effects when inhaled, and pose risks if ingested. Caution is needed due to its flammability.
- What is Isopentane? Aug 12, 2020
Isopentane is an alkane that is butane substituted by a methyl group at position 2. Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressure.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringMethanesulfonic anhydride, or mesyl chloride, stands as a cornerstone in organic chemistry, renowned for its adaptability and broad utility.....
Dec 17,2024Organic Chemistry2-Methylbutane
78-78-4You may like
- 2-Methylbutane
-

- $10.00 / 1KG
- 2025-12-11
- CAS:78-78-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- 2-Methylbutane
-

- $23.00 / 1L
- 2025-01-16
- CAS:78-78-4
- Min. Order: 1L
- Purity: 0.99
- Supply Ability: 3000000tons
- 2-Methylbutane
-
- $1.10 / 1g
- 2023-07-27
- CAS:78-78-4
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons