Versatile Reactivity and Synthetic Potential of Pentachloropyridine in Organic Chemistry
Dec 4,2024
General Description
Pentachloropyridine is a key compound in organic synthesis, offering versatile reactivity for diverse chemical applications. Its synthesis, pioneered by Kekule and enhanced by Sell and Dootson, allows for further functionalization with controlled regioselectivity. Pentachloropyridine's reactivity, highlighted in various studies, showcases its potential in nucleophilic substitution reactions, leading to different product outcomes based on the choice of nucleophile and solvent. These reactions yield valuable derivatives with applications in medicinal chemistry and organic synthesis. Overall, pentachloropyridine serves as a valuable building block for designing novel compounds, offering researchers a pathway to tailor-made functionalization strategies in organic chemistry.

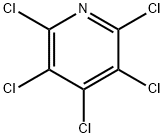
Figure 1. Pentachloropyridine
Synthesis
The first realistic and readily scaled synthesis of penta‑chloropyridine was reported by Kekule, and it is important to note that the report about this work has never been written. Alternatively, commercially available pentachloropyridine and its derivatives can be further functionalized by Sell and Dootson. By using this methodology, the only by‑product, HCl, is spontaneously eliminated from the reaction mixture, by allowing to cool and opened from time to time of the tube to the escape of hydrogen chloride. Another popular method for functionalization of pentachloropyridine was represented in low overall yield by heating a mixture of pyridine at 250 °C, and with using an excess of phosphorus penta‑chloride, the mixture of products can be formed. Different approaches for the synthesis of pentachloropyridine are sumuraized in Scheme 1.1

Scheme 1 Different approaches for the synthesis of pentachloropyridine
Reactivity
Pentachloropyridine exhibits a versatile reactivity profile driven by the substitution of chlorine atoms through aromatic nucleophilic reactions. This unique chemical structure gives rise to a wide array of reactions, making it a valuable starting material in various synthetic pathways. Extensive research has highlighted the reactivity of pentachloropyridine, with multiple studies documenting its behavior in different reaction mechanisms such as AE, SN(ANRORC), EA, and SRN1. One key aspect of its reactivity lies in the differentiation exhibited by perchlorinated heteroaromatic compounds towards nucleophiles, particularly when substituted at the 2-, 3-, and 4-positions of the pyridine ring. The regioselectivity of nucleophilic substitution on pentachloropyridine is attributed to the ortho and para positions of the nitrogen atom within the pyridine ring. Furthermore, the selectivity of nucleophilic substitution at the 3-position can be effectively controlled using strong bases or metal catalysis, inducing EA mechanisms. This selectivity opens up opportunities for targeted functionalization and tailored synthesis strategies utilizing pentachloropyridine 3 as a key building block in organic chemistry. Through systematic investigations and documented reactions, the reactivity of pentachloropyridine continues to be a subject of significant interest and exploration in the field of chemical synthesis. 2
Nucleophilic substitution reactions
Nucleophilic substitution reactions of pentachloropyridine involve the reaction of pentachloropyridine with various N-centered nucleophiles, resulting in substitution at either the 4-position or the 2-position. When reacting with amines such as ammonia, piperidine, and diethylamine, pentachloropyridine yields substitution products at the 4-position with yields ranging from 1% to 70%, or at the 2-position with yields ranging from 30% to 99%. The choice of solvent plays a crucial role in the outcome of the reaction. Aprotic solvents promote 2-substitution, while protic solvents lead to functionalized products in excellent yield. Furthermore, the presence of substitution in the 4-position has a significant effect on accelerating the reaction. Pentachloropyridine has been shown to react efficiently with aliphatic amines such as piperidine and morpholine, yielding predominant forms of 4-alkylaminotetrachloropyridines along with corresponding 2,4-derivatives. Additionally, aromatic amines in dimethylformamide, in the presence of sodium carbonate, have been employed to prepare 4-arylaminotetrachloropyridine in moderate-to-good yields. The reaction of pentachloropyridine with di-functional nucleophiles such as ethane-1,2-diamine or dodecane-1,12-diamine, has resulted in the formation of respective products in low-to-moderate yields. These compounds are considered useful due to their potential biological activity and as starting materials for further intramolecular nucleophilic substitutions to synthesize novel heterocyclic systems. In summary, the nucleophilic substitution reactions of pentachloropyridine exhibit diverse reactivity patterns and product outcomes, influenced by the choice of nucleophile, solvent, and the position of substitution on the pyridine ring. These reactions offer valuable pathways for the synthesis of various substituted pyridine derivatives with potential applications in organic synthesis and medicinal chemistry. 3
Reference
1. Reza RK, Hossein M, Tayebeh D. Utility of pentachloropyridine in organic synthesis. Journal of the Iranian Chemical Society. 2020; 17(9) 2145-2178.
2. Spitzner D. Science of Synthesis. 2004; 15: 11-284.
3. Iddon B, Suschitzky H. Polychloroaromatic Compounds. 1974. 2: 197-364.
- Related articles
- Related Qustion
- The chemical Properties of Pentachloropyridine. Sep 28, 2023
Pentachloropyridine is a perhalogenated heterocyclic compound with a broad and developing chemistry.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPolyquaternium-10, derived from hydroxyethyl cellulose, offers conditioning benefits in skincare and haircare, with a novel detection method ensuring accurate levels in cosmetic products.....
Apr 3,2024APIPentachloropyridine
2176-62-7You may like
Pentachloropyridine manufacturers
- Pentachloropyridine
-

- $10.00 / 1KG
- 2025-12-11
- CAS:2176-62-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Pentachloropyridine
-

- $0.00 / 20kg
- 2025-12-11
- CAS:2176-62-7
- Min. Order: 1kg
- Purity: 99%min
- Supply Ability: 100kgs
- Pentachloropyridine
-
- $1.10 / 1g
- 2025-11-18
- CAS:2176-62-7
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons min