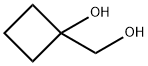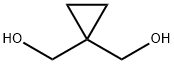
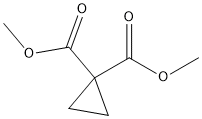
1,1-Bis(hydroxymethyl)cyclopropane synthesis
- Product Name:1,1-Bis(hydroxymethyl)cyclopropane
- CAS Number:39590-81-3
- Molecular formula:C5H10O2
- Molecular Weight:102.13
6914-71-2

39590-81-3
The general procedure for the synthesis of 1,1-cyclopropanedimethanol from dimethyl 1,1-cyclopropanedicarboxylate was as follows: lithium aluminum hydride (LiAlH4, 569 mg, 15.0 mmol) was added batchwise to a solution of dimethyl-1,1-cyclopropanedicarboxylate (791 mg, 5.01 mmol) in ether (Et2O, 20 mL) at 0 °C. The reaction mixture was stirred at room temperature for 4 h. The reaction was subsequently quenched with saturated sodium sulfate (Na2SO4) solution at 0 °C. The precipitated solid was filtered and washed with tetrahydrofuran (THF). The filtrate was concentrated and purified by column chromatography (eluent: ethyl acetate, EtOAc) to afford 440 mg (86% yield) of the target product 1,1-cyclopropanedimethanol. The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 4.02 (s, 2H), 3.56 (s, 4H), 0.48 (s, 4H); mass spectrum (electrospray ionization, ES) m/z: 125 ([M + Na]+).
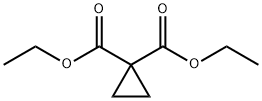
1559-02-0
323 suppliers
$5.00/5g

7732-18-5
507 suppliers
$12.69/100ml

39590-81-3
291 suppliers
$10.00/5g
Yield:39590-81-3 81 g (0.79 mol, 84%)
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran
Steps:
17.7 Step 7
Step 7 1,1-cyclopropanedimethanol A solution of lithium aluminum hydride (50 g, 1.32 mol) in 1.6L of THF was cooled to -18° C. under N2. A solution of diethyl 1,1-cyclopropanedicarboxylate (175 g, 0.94 mol) in 1.2L of THF was then added dropwise over 50 min, at such a rate that the internal temperature of the reaction remained below 10° C. The cooling bath was then removed, and after 15 min, the temperature had reached 15° C. The reaction was then quenched by careful addition of 50 mL H2 O, followed by 50 mL of 15% NaOH, and then 150 mL of H2 O. After the mixture turned white, it was filtered through celite, and the bed was washed with 4L of THF. Evaporation gave an oil which was distilled to give 81 g (0.79 mol, 84%) of the title compound as a colorless oil, b.p. 131°-138° C./15 mm Hg. 1 H NMR (CDCl3) δ0.48 (4H, s), 3.30 (2H, s), 3.58 (4H, s).
References:
Merck Frosst Canada, Inc. US5212180, 1993, A

6914-71-2
210 suppliers
$35.00/25 g

39590-81-3
291 suppliers
$10.00/5g

1559-02-0
323 suppliers
$5.00/5g

39590-81-3
291 suppliers
$10.00/5g
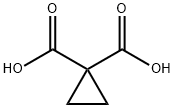
598-10-7
360 suppliers
$7.00/1g

39590-81-3
291 suppliers
$10.00/5g