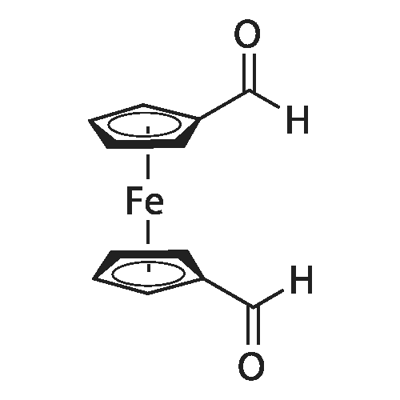
1,1'-FERROCENEDICARBOXALDEHYDE synthesis
- Product Name:1,1'-FERROCENEDICARBOXALDEHYDE
- CAS Number:1271-48-3
- Molecular formula:C12H10FeO210*
- Molecular Weight:242.05
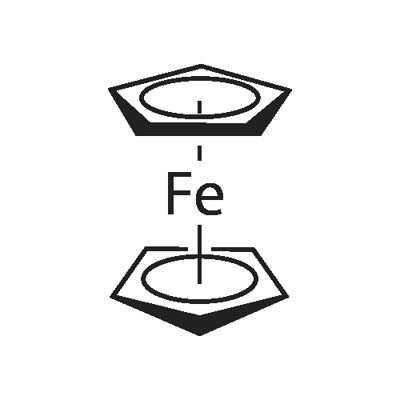
102-54-5

68-12-2

1271-48-3
The general procedure for the synthesis of 1,1'-ferrocenedicarboxaldehyde from ferrocene and N,N-dimethylformamide, based on a modification of the literature procedure [26b], was as follows: ferrocene (10 g, 0.054 mol) was dissolved in anhydrous hexanes (200 mL) at room temperature, n-butyllithium solution (67 mL, 1.6 M, 0.10 mol) was added slowly and dropwise, followed by the addition of tetramethylene methyl ethylenediamine (TMEDA) (14.7 g, 0.25 mol). It was shown that the addition of the reactants at -50 °C had no significant effect on the yield. After stirring the reaction mixture for 18 h at room temperature, the dilithiated ferrocene precipitated as an orange solid. The suspension was cooled to 0 °C, N,N-dimethylformamide (DMF) (7.85 g, 0.11 mol, 8.3 mL) was added and stirring was continued for 2 h at room temperature.
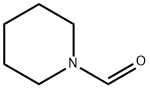
2591-86-8
296 suppliers
$14.00/5g

102-54-5
505 suppliers
$5.00/25g

1271-48-3
117 suppliers
$45.00/100mg
Yield:1271-48-3 80%
Reaction Conditions:
with n-butyllithium;N,N,N,N,-tetramethylethylenediamine in diethyl ether;hexaneAr; ferrocene (53.8 mmol) suspnd. in hexane, hexane soln. of BuLi (107.5mmol) added, TMEDA (55.7 mmol) added dropwise, stirred at room temp. fo r 14 h, Et2O soln. of ligand (104.1 mmol) added dropwise, stirred for 30min; extd. (H2O), reextd. (CH2Cl2), org. layer washed (aq. HCl), dried (MgSO4), filtered, concd., chromd. (SiO2, CH2Cl2/hexane=1/1), dried;
References:
Barry, Kevin P.;Nataro, Chip [Inorganica Chimica Acta,2009,vol. 362,# 6,p. 2068 - 2070]

102-54-5
505 suppliers
$5.00/25g

1271-48-3
117 suppliers
$45.00/100mg

102-54-5
505 suppliers
$5.00/25g
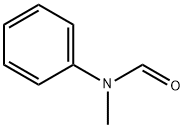
93-61-8
301 suppliers
$10.00/5 g
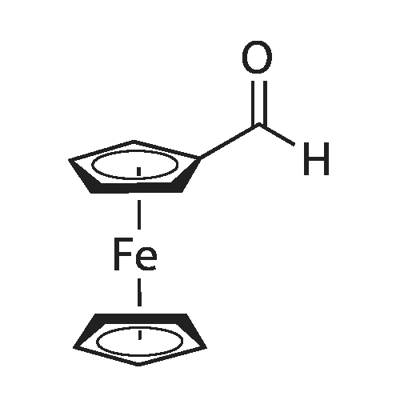
12093-10-6
191 suppliers
$8.00/1g

1271-48-3
117 suppliers
$45.00/100mg