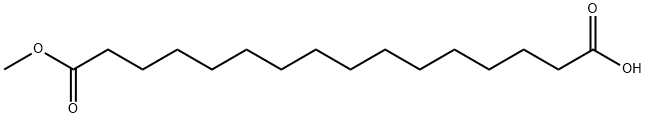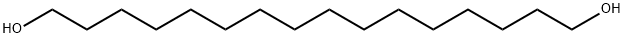
1,16-HEXADECANEDIOL synthesis
- Product Name:1,16-HEXADECANEDIOL
- CAS Number:7735-42-4
- Molecular formula:C16H34O2
- Molecular Weight:258.44
506-13-8
149 suppliers
$37.00/1g
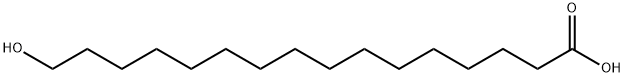
109-29-5
166 suppliers
$50.00/2 g
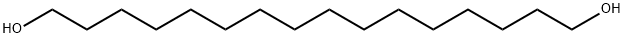
7735-42-4
132 suppliers
$45.00/10mg
Yield:7735-42-4 76% ,109-29-5 10%
Reaction Conditions:
with [ReOCl2(1,2-bis(diphenylphosphino)ethane)];hydrogen;potassium tetraphenylborate in toluene at 150; under 30003 Torr; for 24 h;Autoclave;Inert atmosphere;
Steps:
8
General procedure: By the following method, it went 3-reduction of phenylpropionic acid (hydrogenation).Put a stir bar in a dry glass tube (25mL), further, 3-phenylpropionic acid (75.09mg, 0.5mmol), the rhenium complex 4 (7.07mg, 0.010mmol), potassium tetraphenylborate (17.92mg , accommodates 0.05 mmol), the tubes containing this mixture, was inserted into the autoclave. Then, after replacing the inside of the autoclave in an argon gas atmosphere was added while continuing flow of argon gas dehydration toluene (4.0mL). This autoclave through a stainless steel tube by introducing hydrogen gas from a hydrogen gas cylinder connected, the inside of the autoclave was replaced with hydrogen gas, then disconnect the hydrogen gas pressure from the leak valve. This operation - was repeated (substituted de substitution) five times. Finally, the hydrogen gas pressure in the autoclave was set to 4 MPa, using a constant temperature bath, and allowed to react for 12 hours at 180 ° C. After completion of the reaction, the autoclave was cooled by immersion in an ice bath, almost to room temperature. Then, carefully release the hydrogen gas that is inside in the draft. After removing the solvent, the reaction product was analyzed by 1H NMR using mesitylene (60.1 mg, 0.5 mmol) as an internal standard substance. As a result, 3-phenylpropyl alcohol, and 3-phenylpropionic acid 3-phenylpropyl The yield was 98% and 1%, respectively. In the above Examples 6-1,Substrate (carboxylic acid compounds), and hydrogenation conditions (hydrogen pressure),Except that to adopt the conditions described in Table 7-9,It has been reduced (hydrogenated) in the same manner as in Example 6-1.However,The entry 19-26 in Table 9,Using tetrahydrofuran (THF) as a solvent.The results are shown in Tables 7 to 9.
References:
Nagoya University;Saito, Susumu;Noyori, Ryoji;Santosh, Agrawal;Naruto, Masayuki JP2015/124156, 2015, A Location in patent:Paragraph 0117; 0118; 0127; 0129; 0130

109-29-5
166 suppliers
$50.00/2 g
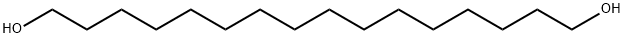
7735-42-4
132 suppliers
$45.00/10mg
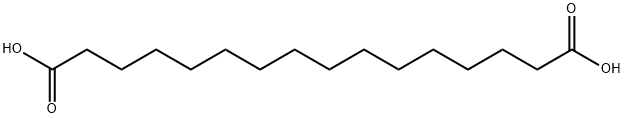
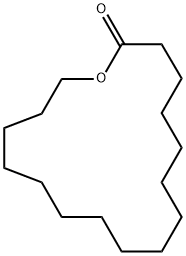
505-54-4
361 suppliers
$5.00/100mg
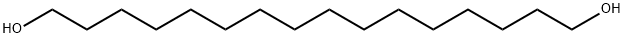
7735-42-4
132 suppliers
$45.00/10mg
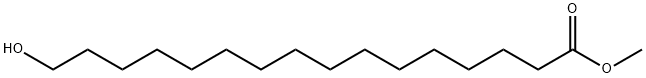
36575-67-4
14 suppliers
inquiry
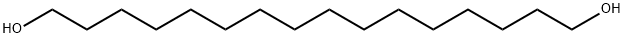
7735-42-4
132 suppliers
$45.00/10mg