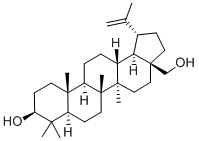
Betulin synthesis
- Product Name:Betulin
- CAS Number:473-98-3
- Molecular formula:C30H50O2
- Molecular Weight:442.72
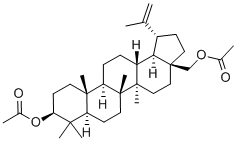
1721-69-3

473-98-3
General procedure for the synthesis of high purity betulinol from betulin diacetate: In a glass reactor equipped with a mechanical stirrer, a reflux condenser, an air inlet and an exhaust valve, 2.00 kg of betulin diacetate of Example 5 was dissolved in a mixture of 10 L of toluene and 8 L of ethanol containing 700 g of potassium hydroxide. The reaction mixture was refluxed under stirring for 2 hours and subsequently filtered through a polypropylene (PP) filter cloth while hot. The filtrate was inoculated with pure betulinol crystals and after cooling, the crystallized betulinol was collected on a process filter and washed sequentially with 3 L of ethanol, 3 L of 50% ethanol, and 30 L of boiling water and finally with 5 L of boiling distilled water. Crystallized betulinol was dried in a hot air oven at 70-75 °C for 48 h to give 1.12 kg (66% yield) of finely crystallized powdered betulinol with a melting point of 256-258 °C, [α]D +16 (10% v/v CHCl3 solution of CH3OH; 0.35 g/100 mL), and HPLC purity of 98.9-99.1%. The 1H NMR and 13C NMR spectral data of the resulting birch alcohol, determined by 10% v/v CDCl3 solution of CD3OD, were consistent with the literature. The mother liquor was concentrated to 8 L and then inoculated and cooled to give a second portion of crystalline betulinol, which was washed and dried in the same manner to give 0.46 kg (27% yield) of betulinol with a melting point of 256-257 °C, [α]D +16 (10% v/v CHCl3 solution of CH3OH; 0.35 g/100 mL), and an HPLC purity of 98.7-99.0%. The total yield was 1.58 kg birch alcohol (93%).
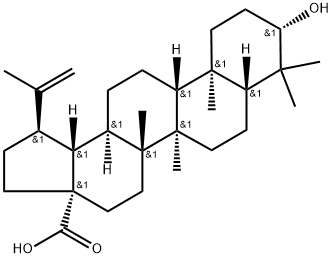
472-15-1
456 suppliers
$5.00/25mg

473-98-3
433 suppliers
$16.35/10mg
Yield:473-98-3 90%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran for 5 h;Reflux;Inert atmosphere;Sealed tube;
Steps:
4.4. Reduction of C-28 carboxylic acid group of 1, 8, and 9
General procedure: To a dried 25 mL glass vial equipped with a magnetic stirrer, containing13.7 mg (0.03 mmol) of (+)-ursolic acid (1), 2 mL of a 0.02Msolution of LiAlH4 in dried THF (0.04 mmol) were added to the vialunder argon. The vial was sealed, and the mixture was stirred withreflux for 5 h, it was cooled to room temperature. Next, the reactionmixture was diluted with aqueous ether, extracted with CH2Cl2 (DCM),dried over anhydrous Na2SO4, evaporated to dryness and chromatographedover silica gel to obtain 12.0 mg (0.027 mmol) of the product,(+)-uvaol (10), with a reaction yield of 90.9%. Following this sameprocedure, 12.0 mg (0.03 mmol) of the product (+)-erythrodiol (11,with a yield of 90.9%) were prepared from 13.7 mg (0.03 mmol) of(+)-oleanolic acid (8, purchased from Sigma), and 7.9 mg(0.018 mmol) of the product (+)-betulin (12) (with a yield of 90.0%)were prepared from 9.1 mg (0.02 mmol) of (+)-betulinic acid (9, isolatedfrom our previous study).
References:
Ren, Yulin;Anaya-Eugenio, Gerardo D.;Czarnecki, Austin A.;Ninh, Tran Ngoc;Yuan, Chunhua;Chai, Hee-Byung;Soejarto, Djaja D.;Burdette, Joanna E.;de Blanco, Esperanza J. Carcache;Kinghorn, A. Douglas [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 15,p. 4452 - 4460] Location in patent:supporting information

1721-69-3
55 suppliers
$58.00/10 mg

473-98-3
433 suppliers
$16.35/10mg

1721-69-3
55 suppliers
$58.00/10 mg

473-98-3
433 suppliers
$16.35/10mg
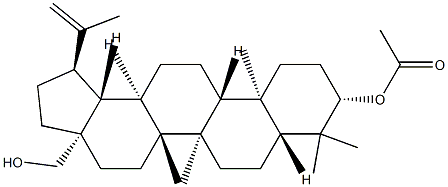
27570-20-3
8 suppliers
inquiry