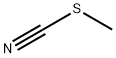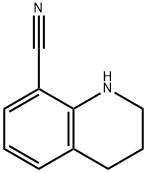
1,2,3,4-tetrahydro-8-Quinolinecarbonitrile synthesis
- Product Name:1,2,3,4-tetrahydro-8-Quinolinecarbonitrile
- CAS Number:50741-37-2
- Molecular formula:C10H10N2
- Molecular Weight:158.2
635-46-1
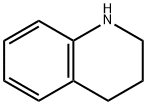
545-06-2

50741-37-2
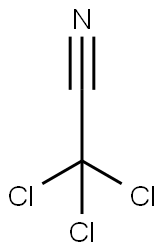
Under cooling conditions in an ice bath, 1.33 g of 1,2,3,4-tetrahydroquinoline was dissolved in 10 mL of toluene, followed by the slow addition of 5.5 mL of a 2.02 M toluene solution of boron trichloride. The reaction mixture was placed in an oil bath and refluxed for 1 hour. Upon completion of the reaction, the toluene solvent was removed by atmospheric pressure distillation. The resulting residue was mixed with 2 mL of trichloroacetonitrile and heated in an oil bath at 60°C to 62°C for 20 hours. The reaction mixture was cooled, dissolved in 20 ml of dichloromethane and slowly poured into 40 ml of methanol solution containing 9.1 g of potassium carbonate under cooling in an ice bath. This mixture was refluxed in an oil bath for 1 hour and subsequently filtered to remove insoluble impurities. The filtrate was concentrated and subjected to liquid-liquid partition extraction with water and dichloromethane. The dichloromethane layer was washed with dilute hydrochloric acid, dried over anhydrous magnesium sulfate and subsequently concentrated to remove the solvent. The resulting crude product (1.53 g) was purified by Lobar column chromatography employing dichloromethane as eluent and the target fractions were collected. The product was further recrystallized using a mixed ether-hexane solvent, resulting in 1.07 g of 8-cyano-1,2,3,4-tetrahydroquinoline crystals with a melting point of 75°C to 76°C in 68% yield.
Yield:50741-37-2 68%
Reaction Conditions:
with boron trichloride;potassium carbonate in methanol;dichloromethane;toluene;
Steps:
74 EXAMPLE 74
EXAMPLE 74 To a solution of 1.33 g of 1,2,3,4-tetrahydroquinoline in 10 ml of toluene was added 5.5 ml of a solution of 2.02M of boron trichloride in toluene under ice-cooling. The mixture was refluxed on an oil bath for 1 hr. and evaporated under atmospheric pressure to remove the toluene. The residue was mixed with 2 ml of trichloroacetonitrile and heated at 60°-62° C. on an oil bath for 20 hr. The reaction mixture was dissolved in 20 ml of methylene chloride and poured into a mixture of 9.1 g of potassium carbonate and 40 ml of methanol under ice-cooling. The mixture was refluxed on an oil bath for 1 hr. and filtered to remove the insoluble material. The filtrate was concentrated and partitioned between water and methylene chloride. The methylene chloride layer was washed with dilute HCl, dried over anhydrous magnesium sulfate and concentrated to remove the solvent. The residue (1.53 g) was purified on a Lobar column and the product (1.10 g) from the methylene chloride elude was recrystallized from ether-n-hexane to give 1.07 g of 8-cyano-1,2,3,4-tetrahydroquinoline as crystals melting at 75°-76° C. Yield: 68%
References:
US4774331,1988,A
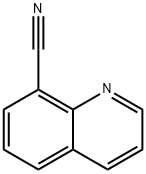
35509-27-4
53 suppliers
inquiry

50741-37-2
43 suppliers
inquiry
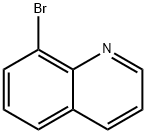
16567-18-3
315 suppliers
$6.00/1g

50741-37-2
43 suppliers
inquiry