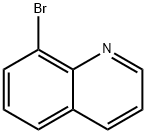
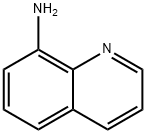
8-Bromoquinoline synthesis
- Product Name:8-Bromoquinoline
- CAS Number:16567-18-3
- Molecular formula:C9H6BrN
- Molecular Weight:208.05
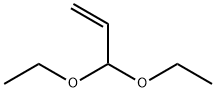
3054-95-3
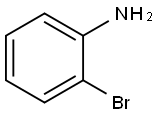
615-36-1

16567-18-3
GENERAL PROCEDURE: A 1N HCl solution (82.5 mL) was added to a round-bottomed flask containing o-bromoaniline (~1 mmol). Acrolein diethyl acetal (2.5 mmol) was then added. The reaction mixture was refluxed at 111 °C for 24 hours. After completion of the reaction, it was cooled to room temperature and neutralized to pH 7-8 with solid Na2CO3. The product was extracted with dichloromethane (3 x 100 mL), the organic layers were combined and dried over anhydrous Na2SO4. The solvent was removed by evaporation under reduced pressure to give the crude product. The crude product was purified by column chromatography using a solvent mixture of hexane and ethyl acetate (or 15% ethyl acetate/cyclohexane and methanol) as eluent to give the target product 8-bromoquinoline.
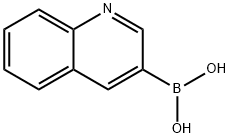
86-58-8
290 suppliers
$15.00/1g

16567-18-3
313 suppliers
$6.00/1g
Yield:16567-18-3 90%
Reaction Conditions:
with tetrabutylammomium bromide;copper(ll) bromide in water at 100;Sealed tube;
Steps:
1. Preparation of aryl bromides
General procedure: To a stirred solution of arylboronic acids 1 (1 mmol), CuBr2 (335 mg, 1.5 mmol), TBAB (33 mg, 0.1 mmol) water (5mL) in a sealed tube for 5-12 h at 100 °C. After completion of the reaction as indicated by TLC, the mixture was extracted with EtOAc (2×5 mL). The organic layer was dried by anhydrous Na2SO4, concentrated in vacuo, and the residue was isolated by simply column chromatography (PE/EA 40:1-20:1) to afford products, respectively.
References:
Tang, Yan-Ling;Xia, Xian-Song;Gao, Jin-Chun;Li, Min-Xin;Mao, Ze-Wei [Tetrahedron Letters,2021,vol. 64,art. no. 152738] Location in patent:supporting information
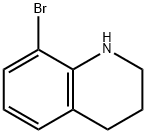
937640-02-3
34 suppliers
inquiry

16567-18-3
313 suppliers
$6.00/1g
578-66-5
326 suppliers
$6.00/1g

16567-18-3
313 suppliers
$6.00/1g

3054-95-3
208 suppliers
$28.00/25g

615-36-1
415 suppliers
$10.00/5g

16567-18-3
313 suppliers
$6.00/1g
