
1-(2,3-DIHYDRO-BENZOFURAN-2-YLMETHYL)-PIPERAZINE synthesis
- Product Name:1-(2,3-DIHYDRO-BENZOFURAN-2-YLMETHYL)-PIPERAZINE
- CAS Number:876717-03-2
- Molecular formula:C13H18N2O
- Molecular Weight:218.29
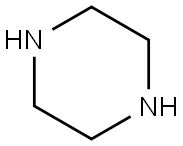
110-85-0
589 suppliers
$5.00/5G
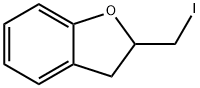
59152-49-7
11 suppliers
$165.00/100mg

876717-03-2
10 suppliers
$400.00/1g
Yield:876717-03-2 54%
Reaction Conditions:
with potassium carbonate in acetonitrile; for 2 h;Reflux;
Steps:
12.4 Step 4. Formation of 1-(2,3-dihydro-1-benzofuran-2-ylmethyl)piperazine
0489] To a solution of 2-(iodomethyl)-2,3-dihydro-1-benzofuran (5.3 g, 20.4 mmol) in acetonitrile (70 ml) was added potassium carbonate (5.6 g, 40.5 mmol, 2 eq) and piperazine (8.8 g, 102.2 mmol, 5 eq). The resulting mixture was heated at reflux for 2 hours. The solids were then filtered off and the filtrate was concentrated under vacuum. The crude material was purified by silica gel chromatography using 0.5-2.5% methanol in dichloromethane to elute. The product-containing fractions were combined and concentrated under vacuum to afford 1-(2,3-dihydro-1-benzofuran-2-ylmethyl)piperazine as brown oil (2.4 g, 54%); (ES, m/z): [M+H]+ 219; 1H NMR (300 MHz, CDCl3): δ 7.07-7.17 (m, 2H), 6.78-6.85 (m, 2H), 4.92-5.01 (m, 1H), 3.22 (dd, J=9.0 Hz, 15.6 Hz, 1H), 2.91-2.99 (m, 4H), 2.71-2.82 (m, 1H), 2.53-2.71 (m, 6H).
References:
US2014/142114,2014,A1 Location in patent:Paragraph 0488; 0489
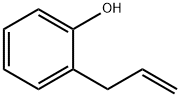
1745-81-9
302 suppliers
$14.00/5g

876717-03-2
10 suppliers
$400.00/1g
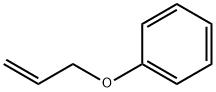
1746-13-0
243 suppliers
$9.00/5g

876717-03-2
10 suppliers
$400.00/1g
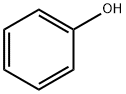
108-95-2
785 suppliers
$14.00/25g

876717-03-2
10 suppliers
$400.00/1g