[1,2,4]triazolo[1,5-a]pyridin-2-ylmethanol synthesis
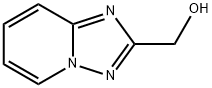
- Product Name:[1,2,4]triazolo[1,5-a]pyridin-2-ylmethanol
- CAS Number:265643-91-2
- Molecular formula:C7H7N3O
- Molecular Weight:149.15
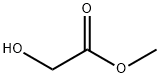
![ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate](/CAS2/GIF/62135-58-4.gif)
62135-58-4
37 suppliers
$45.00/10mg
![[1,2,4]triazolo[1,5-a]pyridin-2-ylmethanol](/CAS/20180703/GIF/265643-91-2.gif)
265643-91-2
28 suppliers
inquiry
Yield:265643-91-2 91%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran at 65; for 0.25 h;Inert atmosphere;
Steps:
64.4 Step 4:
[1,2,4]Triazolo[1,5-a]pyridin-2-ylmethanol
Under argon atmosphere, ethyl[1,2,4]triazolo[1,5-a]pyridine-2-carboxylate (1 g, 5.23 mmol) was combined with THF (10 mL) at RT to give a brown suspension.
Sodium borohydride (1.19 g, 31.4 mmol) was added in four portions.
The mixture was heated to 65° C. for 15 min.
After cooling down to RT, ethanol (10 mL) was added dropwise over a period of 15 min.
The mixture was stirred at 65° C. for 4 h.
The mixture was cooled down to 0-5° C. and NH4Cl (saturated aqueous solution, 20 mL) was added dropwise over a period of 10 min (foam.).
Water (20 mL) was added and the yellow suspension was poured into dichloromethane (100 mL) and extracted with dichloromethane (4*75 mL).
The combined organic layer was dried over MgSO4 and concentrated in vacuo to give the product as light yellow solid (720 mg, 4.76 mmol, 91%) which was used without further purification for the next step.
MS: M=150.1 (M+H)+
References:
US2013/59833,2013,A1 Location in patent:Paragraph 0517-0518
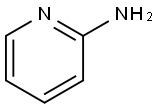
504-29-0
555 suppliers
$5.00/5G
![[1,2,4]triazolo[1,5-a]pyridin-2-ylmethanol](/CAS/20180703/GIF/265643-91-2.gif)
265643-91-2
28 suppliers
inquiry