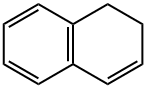
1,2-DIHYDRONAPHTHALENE synthesis
- Product Name:1,2-DIHYDRONAPHTHALENE
- CAS Number:447-53-0
- Molecular formula:C10H10
- Molecular Weight:130.19
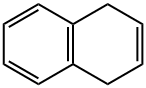
612-17-9
94 suppliers
$22.00/100mg

447-53-0
129 suppliers
$12.00/1g
Yield:447-53-0 76%
Reaction Conditions:
with manganese;6,6'-dimethyl-2,2'-bipyridine;phosphonic acid diethyl ester;nickel dibromide in chloroform at 35; for 24 h;
Steps:
20 Synthesis of Example 20
According to the general operating procedures, 1,4-dihydronaphthalene (1t) (130.1 mg, 1.0 mmol) is used as a raw material, 0.50 mol% nickel bromide (1.1 mg, 0.005 mmol) is used as a catalyst, and 0.60 mol% 6,6'- Dimethyl-2,2'-bipyridine (1.0 mg, 0.006 mmol) is the ligand, manganese powder (16.5 mg, 0.30 mmol, 0.30 equiv) is the reducing agent, and diethyl phosphite (30.0 mg, 0.20 mmol, 0.20equiv) was a hydrogen source, and anhydrous chloroform (1.0 mL) was used as a solvent for the reaction.The reaction system was stirred at 35 ° C. for 24 hours. A colorless oil (99.5 mg) was obtained by silica gel column chromatography (n-hexane) with an isolated yield of 76%
References:
Nanjing University;Zhu Shaolin;Ye Feng;Han Bo CN110878012, 2020, A Location in patent:Page/Page column 12
529-33-9
193 suppliers
$6.00/250mg

447-53-0
129 suppliers
$12.00/1g
529-34-0
478 suppliers
$6.00/10g

447-53-0
129 suppliers
$12.00/1g
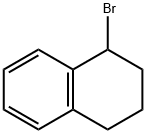
75238-77-6
15 suppliers
inquiry

447-53-0
129 suppliers
$12.00/1g
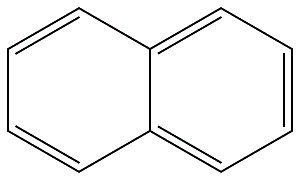
91-20-3
530 suppliers
$13.19/250mg

447-53-0
129 suppliers
$12.00/1g