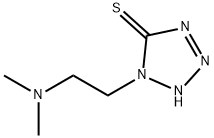
1-[2-(Dimethylamino)ethyl]-1H-tetrazole-5-thiol synthesis
- Product Name:1-[2-(Dimethylamino)ethyl]-1H-tetrazole-5-thiol
- CAS Number:61607-68-9
- Molecular formula:C5H11N5S
- Molecular Weight:173.24
![1-[2-(Dimethylamino)ethyl]-1H-tetrazole-5-thiol 1-[2-(Dimethylamino)ethyl]-1H-tetrazole-5-thiol](/NewsImg/2023-12-01/6383702325966114244664106.jpg)
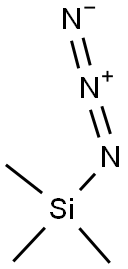
7097-89-4
12 suppliers
inquiry
![1-[2-(Dimethylamino)ethyl]-1H-tetrazole-5-thiol](/CAS/GIF/61607-68-9.gif)
61607-68-9
295 suppliers
$11.00/5g
Yield:61607-68-9 84%
Reaction Conditions:
with sodium azide in water;isopropyl alcohol at 80; for 3 h;
Steps:
1.2
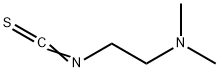
Step 2: Preparation of 1-(2-(dimethylamino)ethyl)-1H-tetrazole-5-thiol [215] 1 mg of N1, N1-dimethlethan-1,2-diamine and 1 mmol of di-2-pyridyl thiamocarbonate were dissolved in dichloromethane for 2 hours and stirred. It was extracted by using 50 ml of ethylacetate and adding water to the reactant. Organic solvents were cleansed by using water (30 ml X 2) and brine (30 ml) and separated. The intermediate of 2-isothiocyanato-N, N-dimethylethanamine was synthesized by removing water using magnesiumsulfat anhydrous from the separated organic solvents and removing ethylacetate through filtration and decompression. 1 mmol of the synthesized 2-isocyanato-N, N-dimethylethanamine and 1 mmol of sodium azide were stirred with 2 ml of 2-propanol and 2 ml of water for 3 hours in the temperature of 80°C. The solvent was removed by reducing pressure after acidification using 2N HCl. The desired form of the compound, 1-(2-dimethylamino)ethyl)-1H-tetrazole-5-thiol was obtained in the yield of 84% by purifying with silica gel column chromatography using methanol and dichloromethane as developing solvents. [216] [217] 1H NMR (CDCl3, 400MHz) 9.05(s, 1H), 8.81(s, 1H), 8.15(s, 1H), 7.76(s, 1H), 4.47(t, 2H, J=6.4Hz), 2.83(t, 2H, J=6.4Hz), 2.19(s, 6H)[218] MS(ESI+) m/z 453.1(M+1)
References:
WO2011/122815,2011,A2 Location in patent:Page/Page column 18
![Carbamodithioic acid, N-[2-(dimethylamino)ethyl]-, methyl ester](/CAS/20210305/GIF/66309-55-5.gif)
66309-55-5
0 suppliers
inquiry
![1-[2-(Dimethylamino)ethyl]-1H-tetrazole-5-thiol](/CAS/GIF/61607-68-9.gif)
61607-68-9
295 suppliers
$11.00/5g