
Ethanol synthesis
- Product Name:Ethanol
- CAS Number:64-17-5
- Molecular formula:C2H6O
- Molecular Weight:46.07
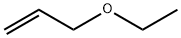
557-31-3
74 suppliers
$54.00/5mL

64-17-5
826 suppliers
$10.00/50g
Yield:64-17-5 100%
Reaction Conditions:
with hexaaquaruthenium(II) tosylate in water-d2 at 50; for 18 h;Inert atmosphere;
Steps:
General procedure: D (11 mg, 51.7 lmol), E (5.5 lL, 51.7 lmol), and F (2.5 lL,51.7 lmol) were each separately dissolved in 0.70 mL D2O with2.0 mg of 1 (3.63 lmol), 7% catalyst. The D2O was previouslysparged with argon for 5 min. Each of the reactions was run ina regular NMR tube at 50 C and monitored using 1H NMRspectroscopy; the tosylate anion of 1 was used as a standard forcalculating the amount of product (i.e.% yield) formed.Confirmation of the final products was done through authenticaddition including the use of uridine from the hydrolysis G andH. For variable temperature runs, 2.0 mg of 1 (3.63 lmol) was dissolvedin 0.70 mL D2O (previously sparged with Ar) and equilibratedat the desired temperatures (30 C, 40 C, 60 C) for at least 15 min inthe NMR magnet. The immediate addition of 5.5 lL of ethyleneglycol monoallyl ether, A, (52 lmol) to this NMR tube was followedby spectral acquisitions at the set temperature.
References:
Kuo, Louis Y.;Delaney, Frances E. [Inorganica Chimica Acta,2015,vol. 435,p. 335 - 339]
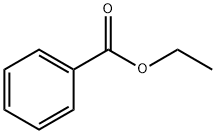
93-89-0
527 suppliers
$13.00/100g

64-17-5
826 suppliers
$10.00/50g

100-51-6
1413 suppliers
$5.00/100g

141-78-6
685 suppliers
$22.37/250ml

64-17-5
826 suppliers
$10.00/50g
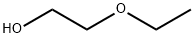
110-80-5
535 suppliers
$10.00/25ml

64-17-5
826 suppliers
$10.00/50g

64-19-7
1626 suppliers
$10.00/25ML

64-17-5
826 suppliers
$10.00/50g