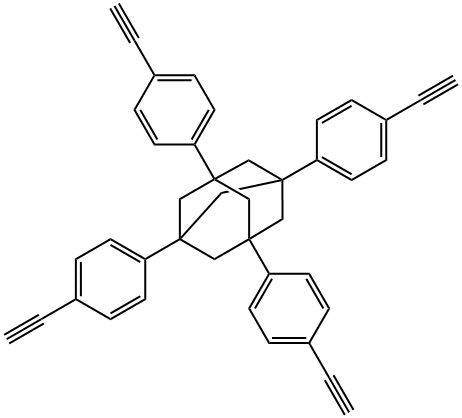
1,3,5,7-tetrakis(4-ethinylphenyl)adamantane synthesis
- Product Name:1,3,5,7-tetrakis(4-ethinylphenyl)adamantane
- CAS Number:144970-32-1
- Molecular formula:C42H32
- Molecular Weight:536.7
Yield:144970-32-1 85%
Reaction Conditions:
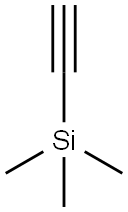
Stage #1: 1,3,5,7-tetrakis(4-iodophenyl)adamantane;trimethylsilylacetylenewith bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide;triethylamine in toluene at 110; for 72 h;Inert atmosphere;
Stage #2: with potassium carbonate in tetrahydrofuran;methanol at 20; for 5 h;
Steps:
1,3,5,7-Tetrakis(4-ethynylphenyl)adamantane (23)
In a 250 mL round-bottomed flask was added 1,3,5,7-tetrakis(4-iodophenyl) adamantane (22; 1.6 g, 1.69 mmol), PdCl2(PPh3)2 (82 mg, 0.11 mmol), CuI (22 mg, 0.11 mmol), ethynyltrimethylsilane (2; 5.0 mL, 35.49 mmol), anhyd toluene (40 mL), Et3N (14 mL, 101.4 mmol) under N2 atmosphere. The reaction mixture was stirred for 72 h at 110 °C, after this time the mixture was filtered through a pad of Celite. The filtrate was concentrated and dissolved in CH2Cl2 (150 mL). The CH2Cl2 solution was washed with aq 10% HCl (100 mL), followed by H2O (100 mL) and brine (100 mL). The organic phase was dried (anhyd Na2SO4) and concentrated to use directly in the next step. To this crude material dissolved in MeOH (60 mL) and THF (60 mL) was added solid K2CO3 (1.21 g, 8.76 mmol). The mixture was stirred for 5 h at RT, then concentrated, and the residue was dissolved in CH2Cl2 (150 mL). The organic phase was washed with H2O (100 mL) and brine (100 mL), dried (anhyd Na2SO4) and concentrated. The residue was purified by column chromatography (PE/CH2Cl2 5:1); yield: 780 mg (85%, over 2 steps); white solid. 1H NMR (500 MHz, CDCl3): = 7.49-7.47 (d, J = 8.0 Hz, 8 H), 7.42-7.40 (d, J = 8.0 Hz, 8 H), 3.05 (s, 4 H), 2.12 (s, 12 H).23
References:
Jin, Xiao-Yang;Wu, Chuan-Shuo;Liu, An-Di;Liu, Li;Cheng, Liang [Synthesis,2022,vol. 54,# 6,p. 1643 - 1651]
![Tricyclo[3.3.1.13,7]decane, 1,3,5,7-tetrakis(4-iodophenyl)-](/CAS/20180808/GIF/144970-30-9.gif)
144970-30-9
42 suppliers
inquiry

144970-32-1
20 suppliers
inquiry
16004-75-4
57 suppliers
inquiry

144970-32-1
20 suppliers
inquiry
768-90-1
412 suppliers
$10.00/5g

144970-32-1
20 suppliers
inquiry
71-43-2
721 suppliers
$11.19/25ML

144970-32-1
20 suppliers
inquiry