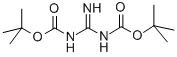
1 3-BIS(TERT-BUTOXYCARBONYL)GUANIDINE synthesis
- Product Name:1 3-BIS(TERT-BUTOXYCARBONYL)GUANIDINE
- CAS Number:154476-57-0
- Molecular formula:C11H21N3O4
- Molecular Weight:259.3

24424-99-5
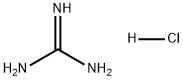
50-01-1
![Carbamic acid, carbonimidoylbis-, bis(1,1-dimethylethyl) ester, [N(E)]- (9CI)](/CAS/20240320/GIF/332882-82-3.gif)
332882-82-3
General procedure for the synthesis of the compound (CAS: 332882-82-3) from di-tert-butyl dicarbonate and guanidinium hydrochloride: guanidinium hydrochloride (12.3 g, 128 mmol) and sodium hydroxide (20.8 g, 519 mmol) were dissolved in water (125 mL) and 1,4-dioxane (250 mL) was added. The mixture was cooled to 0 °C and di-tert-butyl dicarbonate (62.9 g, 288 mmol) was added slowly. The reaction mixture was stirred at room temperature for 16 hours. Upon completion of the reaction, the solution was concentrated under vacuum to one-third of its initial volume. Water (150 mL) was added to the concentrate and extracted with ethyl acetate (3 x 80 mL). The organic phases were combined and washed sequentially with 10% aqueous citric acid (1 × 100 mL), water (1 × 100 mL) and saturated saline (1 × 100 mL). The organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to obtain the crude product. The crude product was purified by rapid chromatography on silica gel (elution gradient: 100% dichloromethane to dichloromethane/methanol 95:5). N,N'-Di-Boc-protected guanidine was finally obtained as a white powder (30.54 g, 91% yield). The structure of the product was confirmed by the following characterization data: 1H NMR (300 MHz, DMSO-d6) δ 10.42 (s, 1H), 8.47 (s, 1H), 1.37 (s, 18H); 13C NMR (75.5 MHz, CDCl3) δ 158.3, 82.3, 28.1; IR (CHCl3) ν 3407, 3124 , 2976, 2930, 1792, 1641, 1549, 1453, 1397, 1365, 1128, 1153 cm-1.

24424-99-5
868 suppliers
$13.50/25G

50-01-1
814 suppliers
$5.00/25g

154476-57-0
82 suppliers
$39.00/1g
Yield:154476-57-0 91%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;lithium hydroxide monohydrate at 0 - 20; for 16 h;
Steps:
1.6 Step 6: Preparation of N,N-di-(tert-butoxycarbonyl)-guanidine as Described in Journal of Organic Chemistry, Vol. 63, No. 23, 1998
24 Guanidine chlorhydrate (7) (12.3 g, 128 mmol) and 25 sodium hydroxide (20.8 g, 519 mmol) were dissolved in 26 water (125 mL), and 27 1,4-dioxane (250 mL) were added. The mixture was cooled to 0° C. and 28 di-tert-butyl-carbonate (62.9 g, 288 mmol) was added. The mixture was allowed to warm at room temperature within 16 h. The solution was concentrated in vacuo to one-third of its initial volume. Water (150 mL) was added to the resulting mixture, and the solution was extracted with ethyl acetate (3×80 mL). The organic phase was then washed with 10% citric acid (1×100 mL), water (1×100 mL) and brine (1×100 mL), dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by flash chromatography on silica gel (eluent: 100% dichloromethane to 11 dichloromethane/12 methanol 95:5). The 29 di-protected guanidine was obtained as a white powder (30.54 g, 91%). 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.42 (s, 1H), 8.47 (s, 1H), 1.37 (s, 18H). 13C NMR (75.5 MHz, CDCl3) δ (ppm) 158.3, 82.3, 28.1. IR (CHCl3) v (cm-1) 3407, 3124, 2976, 2930, 1792, 1641, 1549, 1453, 1397, 1365, 1248, 1153.
References:
US9809621,2017,B2 Location in patent:Page/Page column 31; 32
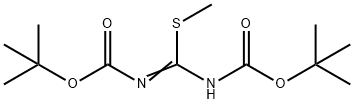
107819-90-9
163 suppliers
$6.00/1g

154476-57-0
82 suppliers
$39.00/1g

24424-99-5
868 suppliers
$13.50/25G

50-01-1
814 suppliers
$5.00/25g

154476-57-0
82 suppliers
$39.00/1g
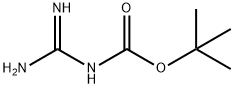
219511-71-4
106 suppliers
$6.00/1g

24424-99-5
868 suppliers
$13.50/25G

50-01-1
814 suppliers
$5.00/25g

154476-57-0
82 suppliers
$39.00/1g
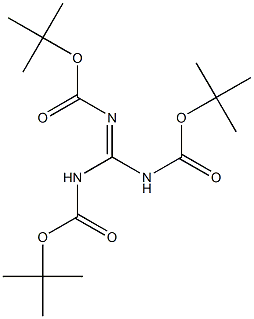
216584-22-4
32 suppliers
$28.00/250MG

24424-99-5
868 suppliers
$13.50/25G

50-01-1
814 suppliers
$5.00/25g

154476-57-0
82 suppliers
$39.00/1g

219511-71-4
106 suppliers
$6.00/1g

216584-22-4
32 suppliers
$28.00/250MG