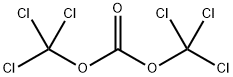
1,3-dichloropropan-2-yl chloroformate synthesis
- Product Name:1,3-dichloropropan-2-yl chloroformate
- CAS Number:55183-48-7
- Molecular formula:C4H5Cl3O2
- Molecular Weight:191.44
Yield:55183-48-7 97%
Reaction Conditions:
with tetrabutyl-ammonium chloride at -30 - 20;
Steps:
2 First step: Synthesis of β-chloro alkylchlorformates
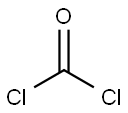
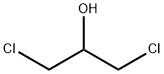
General procedure: Epoxide was charged to a reactor and kept at -30°C. The molar amount of epoxide is listed in Table 1 . 0.01 mol of tetra(n-butyl ammonium chloride were added per 1 mol of epoxide. There- after phosgene is added slowly as the reaction is exothermic. When adding the phosgene the temperature was kept via cooling at the temperature listed in the Table. The time of metering phosgene is listed in the Table. The total amount of phosgene was 1.1 mol per 1 mol of epox- ide. When the addition of phosgene was completed the reaction mixture was further stirred for about (2 hours). Unreacted phosgene was removed by nitrogen stripping. No further work-up was necessary. The obtained β-chloro alkylchlorformates could be used directly in the next step which is the formation of the thiocarbonates.
References:
WO2019/34473,2019,A1 Location in patent:Page/Page column 31-33