
Epichlorohydrin synthesis
- Product Name:Epichlorohydrin
- CAS Number:106-89-8
- Molecular formula:C3H5ClO
- Molecular Weight:92.52
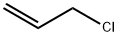
107-05-1
418 suppliers
$16.80/100ML

106-89-8
777 suppliers
$9.00/10g
Yield:106-89-8 99%
Reaction Conditions:
with Cumene hydroperoxide at 60 - 120; under 7500.75 Torr;Temperature;Pressure;
Steps:
4
Using the process shown in Figure 1, 6 reactors are used in series, fresh allyl chloride having a flow rate of 0.5 kg / hr and circulating allyl chloride with 3.5 kg/hr from the top of the reactor 1. Homogeneous molybdenum-based catalyst having a flow rate of 26.7 g/hr from the top of the reactor 1. A cumene solution containing 50% cumene hydroperoxide was mixed with 1 kg / hr of cumene having flow rate of 2 kg / hr from the top entrance of the reactor 6, respectively. The weight ratio of molybdenum to cumene hydroperoxide is 0.0004, allyl chloride: cumene hydroperoxide = 8 (molar ratio). The reaction temperature of the reactor 1 was 60°C, the reaction pressure was 1 MPa, and the conversion of cumene hydroperoxide was 40%. The reaction temperature of the reactor 2 was 80°C, the reaction pressure was 1 MPa, and the conversion of cumene hydroperoxide was 55%. The reaction temperature of the reactor 3 was 90°C, reaction pressire 1 MPa and the conversion of cumene hydroperoxide was 60%. The reaction temperature of the reactor 4 was 100 ° C, reaction pressure was 1MPa, and the conversion of cumene hydroperoxide was 80%. The reaction temperature of the reactor 5 was 110°C, reaction pressure was 1 MPa and the conversion of cumene hydroperoxide was 95%. The reaction temperature of the reactor 6 was 120°C, reaction pressure was 0.1 MPa and the conversion of cumene hydroperoxide was 100%. The streams of allyl chloride, cumene, epichlorohydrin and α,α-dimethylbenzyl alcohol from the bottom of the reactor 6 are separated by distillation column 1, distillation column II and distillation column III, respectively. The operating conditions of the distillation column I were as follows: the number of plates was 50, the pressure was 2 MPa, the column top temperature was 60°C, and the bottom temperature was 150°C. The operating conditions of the distillation column II were 50 plates, a pressure of 2 MPa, a top temperature of 100°C and a bottom temperature of 250°C. The operating conditions of the distillation column III were 50 plates, a pressure of 4 MPa, a top temperature of 130°C, and a bottom temperature of 350°C. The resulting epichlorohydrin product had a flow rate of 0.67 kg / hr, the selectivity of epichlorohydrin to cumene hydroperoxide was 99%, the conversion of cumene hydroperoxide was 100%. The yield of oxychloropropane was 99%.
References:
CN105272946,2016,A Location in patent:Paragraph 0074-0087

56-81-5
1768 suppliers
$5.00/25g

106-89-8
777 suppliers
$9.00/10g
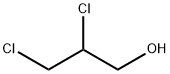
616-23-9
152 suppliers
$42.63/5g

106-89-8
777 suppliers
$9.00/10g
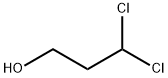
83682-72-8
0 suppliers
inquiry

106-89-8
777 suppliers
$9.00/10g
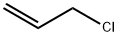
107-05-1
418 suppliers
$16.80/100ML
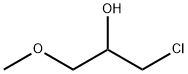
4151-97-7
77 suppliers
$38.00/5g
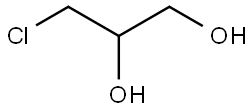
96-24-2
298 suppliers
$11.00/5g

106-89-8
777 suppliers
$9.00/10g