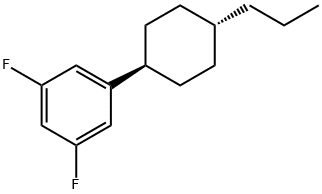
1,3-Difluor-5-(trans-4-propylcyclohexyl)-benzol synthesis
- Product Name:1,3-Difluor-5-(trans-4-propylcyclohexyl)-benzol
- CAS Number:144261-13-2
- Molecular formula:C15H20F2
- Molecular Weight:238.32
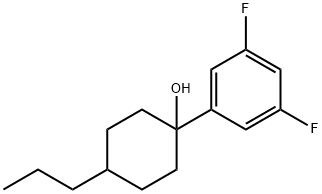
148150-92-9

144261-13-2
Under nitrogen protection, 132 g of crude 1-(1-hydroxy-4-propylcyclohexyl)-3,5-difluorobenzene was dissolved with 2.99 g of p-toluenesulfonic acid monohydrate in 250 mL of toluene. The reaction mixture was heated and refluxed and stirred for 2 hours while the water generated was removed via a water separator. Upon completion of the reaction, the solution was cooled to room temperature and the organic layer was washed with saturated aqueous sodium bicarbonate and saturated saline in turn. The organic layer was dried over anhydrous sodium sulfate and the solvent was removed by distillation under reduced pressure. The residue was completely dissolved in 280 mL of ethyl acetate, and 7.1 g of 5% palladium/carbon catalyst was added, and the reaction was stirred under hydrogen atmosphere (5 MPa pressure) for 6 hours. At the end of the reaction, the palladium catalyst was removed by filtration and the filtrate was distilled under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography. The purified product was dissolved with 3.39 g of potassium tert-butoxide in 350 mL of DMF and the reaction was stirred at room temperature for 4 hours. After the reaction was completed, water and hexane were added for liquid-liquid separation. The aqueous layer was extracted with hexane, the organic layer and hexane extract were combined and washed with saturated saline. The organic layer was dried over anhydrous sodium sulfate and then distilled under reduced pressure to remove the solvent and further distilled at 206 Pa pressure (boiling point 110 °C to 112 °C) to give 58.05 g of 1-(trans-4-propylcyclohexyl)-3,5-difluorobenzene.

148150-92-9
1 suppliers
inquiry

144261-13-2
45 suppliers
$6.00/1g
Yield:144261-13-2 58.05 g
Reaction Conditions:
Stage #1: 1-(1-hydroxy-4-propylcyclohexyl)-3,5-difluorobenzenewith toluene-4-sulfonic acid in toluene; for 2 h;Inert atmosphere;Reflux;
Stage #2: with 5%-palladium/activated carbon;hydrogen in ethyl acetate; under 37503.8 Torr; for 6 h;Inert atmosphere;
Stage #3: with potassium tert-butylate in N,N-dimethyl-formamide at 20; for 4 h;Inert atmosphere;
Steps:
3.3-2
10106] (3-2) In a nitrogen atmosphere, 132 g of crude 1-(1 - hydroxy-4-propylcyclohexyl)-3,5-difluorobenzene and 2.99 g of p-toluenesulfonic monohydrate were dissolved in 250 mE of toluene, and the resultant solution was stirred for 2 hours under reflux while the produced water was removed. Afier the solution was allowed to cool to room temperature, an organic layer was washed with a saturated aqueous sodium bicarbonate solution and saturated brine, and dried by adding sodium sulfate, and then the solvent was distilled off underreduced pressure. Then, the whole amount of the resultantresidue was dissolved in 280 mE of ethyl acetate, and 7.1 g ofpalladium 5%/carbon was added to the solution, followed bystirring in a hydrogen atmosphere at 5 Mpa for 6 hours. After the palladium catalyst was filtered off, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography. Then, the whole amount of the resultant product and 3.39 g of tertiary butoxypotassium were dissolved in 350 mE of DMF, followed by stirring at room temperature for 4 hours. Then, water and hexane were added to separate an organic layet Further, an aqueous layer was subjected to extraction with hexane, and the hexane extract was combined with the organic layer and washed with saturated brine. The resultant mixture was dried by adding sodium sulfate, the solvent was distilled off, and distillation under reduced pressure (206 Pa, b. p.=llO° C. to 112° C.) yielded 58.05 g of 1-(trans-4-propylcyclohexyl)-3, 5 -difluorobenzene.
References:
US2014/275577,2014,A1 Location in patent:Paragraph 0104; 0106
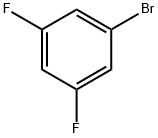
461-96-1
479 suppliers
$6.00/10g

144261-13-2
45 suppliers
$6.00/1g
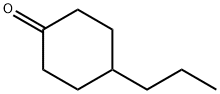
40649-36-3
190 suppliers
$7.00/5g

144261-13-2
45 suppliers
$6.00/1g

148151-00-2
0 suppliers
inquiry

144261-13-2
45 suppliers
$6.00/1g

461-96-1
479 suppliers
$6.00/10g

40649-36-3
190 suppliers
$7.00/5g

144261-13-2
45 suppliers
$6.00/1g