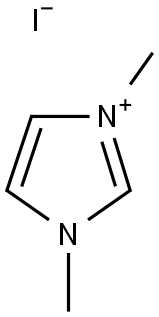
1,3-DIMETHYLIMIDAZOLIUM IODIDE synthesis
- Product Name:1,3-DIMETHYLIMIDAZOLIUM IODIDE
- CAS Number:4333-62-4
- Molecular formula:C5H9IN2
- Molecular Weight:224.0428
5587-42-8
71 suppliers
$121.00/10g

74-88-4
356 suppliers
$15.00/10g

4333-62-4
154 suppliers
$13.00/5g
Yield:4333-62-4 67%
Reaction Conditions:
in tetrahydrofuran for 4 h;Reflux;
Steps:
2.2. Synthesis of RILs
General procedure: Synthesis of the redox anion ferrocenylsulfonyl(trifluoromethylsulfonyl)imide (FcNTf) was performed in accordance with amethod published earlier [14]. All alkylimidazolium cations wereprepared following established procedures, and detailed syntheticsteps for the imidazolium cations [C1C2Im], [C4C4Im], [C2C2Im] and[C1C8Im] are provided in Fig. S1B [31]. In short, a solution of thecorresponding alkylimidazole and alkylhalides was mixed in anacetonitrile solution. The resulting reaction product was washedwith ethyl ether and thefinal product dried under vacuum at 75 Cbefore characterization (yield: 98%). For the synthesis of [C1C1Im]and [C8C8Im], a different procedure (Fig. S1C) was used due to a lowreaction yield of pathway B and some difficulty in eliminatingimpurities from thefinal product. In this case, sodium imidazolatewas added to a 10 ml of THF solution. Two molar equivalents ofalkyl halide (1-iodomethane, bromooctane) were then added tothe mixture. The mixture was maintained under reflux for fourhours. The resulting substance was washed with THF, followed bydichloromethane and diethyl ether (yields: 67% and 99%). Thefinalsteps in RIL synthesis consisted of a recombination reaction ofcation and redox anion from the metathesis reaction with NaFcNTfdissolved in water, carried out in an ultrasonic bath for two hours(Fig. S1D). This step was followed by intensive washing with waterto eliminate the sodium halide salt. The RILs were dried overnightunder vacuum and stored in the glove box for further analysis.Following this drying step, the resulting water concentration inundiluted RIL was found at 144 ppm for [C1C2Im][FcNTf] and170 ppm for the [C1C4Im][FcNTf]. These values are similar to thosereported for vacuum-dried unmodified ionic liquids.
References:
Xie, Han Jin;Gélinas, Bruno;Rochefort, Dominic [Electrochimica Acta,2016,vol. 200,p. 283 - 289]
616-47-7
764 suppliers
$10.00/100g

74-88-4
356 suppliers
$15.00/10g

4333-62-4
154 suppliers
$13.00/5g

616-47-7
764 suppliers
$10.00/100g

616-38-6
721 suppliers
$5.00/5 g

4333-62-4
154 suppliers
$13.00/5g