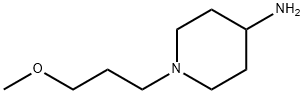
1-(3-Methoxypropyl)-4-piperidinamine synthesis
- Product Name:1-(3-Methoxypropyl)-4-piperidinamine
- CAS Number:179474-79-4
- Molecular formula:C9H20N2O
- Molecular Weight:172.27
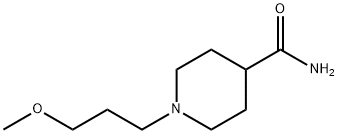
519147-89-8
8 suppliers
inquiry

179474-79-4
171 suppliers
$95.00/1g
Yield:179474-79-4 83%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;potassium hydroxide in water;acetonitrile at 5 - 25; for 13 h;
Steps:
7 Example 7 Synthesis of 1- (3-methoxypropyl) -4-piperidinamine (I)
A solution of 4-formamide-1- (3-methoxypropyl) -piperidine (III) (229 g, 1.14 mol), water (2.06 L) and acetonitrile (571.9 g)After adding KOH (287.3 g, 5.13 mol), the reaction was carried out at a temperature of not higher than 5 ° C by adding dibromohydantoin (180.2 g, 0.63 mol) and the temperature was raised to 15-25 ° C for 13 hours to stop the reaction.The reaction solution was concentrated under reduced pressure at 55 ° C to recover acetonitrile.The residue was extracted with DCM and the organic layer was dried over anhydrous sodium sulfate,And the organic phase was concentrated under reduced pressure at 50 ° C to give 178.2 g of a pale yellow liquid,The yield was 95% (in terms of II-1) and the GC purity was 98.6%.The product was placed in a distillation flask, the pump was decompressed (with a thorned distillation column)External temperature gradient temperature (5 / times),When the internal temperature rose to 72 ~ 73 ,The fractions were collected to give 148 g of colorless transparent liquid,The distillation yield was 83%
References:
CN106146386,2016,A Location in patent:Paragraph 0064; 0065; 0066; 0067
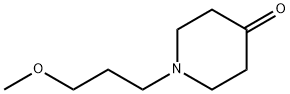
16771-85-0
56 suppliers
$201.96/250mg

179474-79-4
171 suppliers
$95.00/1g
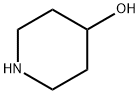
5382-16-1
0 suppliers
$9.00/5G

179474-79-4
171 suppliers
$95.00/1g
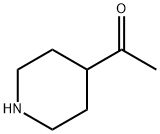
30818-11-2
43 suppliers
inquiry

179474-79-4
171 suppliers
$95.00/1g
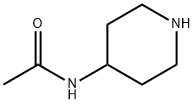
5810-56-0
113 suppliers
$40.95/250mg

179474-79-4
171 suppliers
$95.00/1g