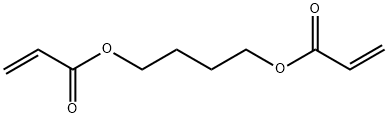
1,4-BUTANEDIOL DIACRYLATE synthesis
- Product Name:1,4-BUTANEDIOL DIACRYLATE
- CAS Number:1070-70-8
- Molecular formula:C10H14O4
- Molecular Weight:198.22
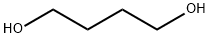
110-63-4
731 suppliers
$24.80/250g
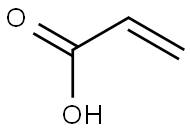
79-10-7
737 suppliers
$20.16/100 mL

1070-70-8
242 suppliers
$5.00/5g
Yield:-
Reaction Conditions:
in toluene at 130; for 4 h;Reagent/catalyst;Temperature;
Steps:
3 Example 3
On the basis of Example 2, the present example provides a solid acid catalyzed synthesis of 1,4-butanediol acrylateMethod, weighed 21.618 g of acrylic acid, 9.012 g of 1,4-butanediol (molar ratio of acrylic acid to 1,4-butanediol of 3: 1)0.451 g of synthetic solid acid catalyst, 1 g of hydroquinone polymerization inhibitor and 5 g of toluene were added to 100 mL of a three-necked flask,On the thermometer and reflux condenser, open the magnetic stirring and heating, set the reaction temperature of 130 oC, the reaction time of 4 hours,To produce 1,4-butanediol diacrylate.The solid acid catalyst was characterized by pyridine infrared and NH3-TPD, indicating that the solid acidThe agent contains both the Lewis acid position and the Brnsted acid site, and contains a large amount of Brnsted strong acid sites and superacid sites.The prepared 1,4-butanediol diacrylate was cooled and sampled, and subjected to nuclear magnetic resonance, gas chromatography-mass spectrometryThe qualitative analysis of the product was carried out by using the gas chromatographic retention time of the standard, and the product was quantitatively analyzed by gas chromatography.As shown in Figure 1 and 2, the retention time 12.3 min is the corresponding chromatographic peak of 1,4-butanediol diacrylate. After distillation under reduced pressureHigh purity 1,4-butanediol diacrylate, the use of gas chromatography quantitative analysis, calculated by the calculation of 1,4-butylThe diol conversion was 99.0% and the selectivity of 1,4-butanediol diacrylate was 96.8%.The solid acid catalyst used in this example was synthesized as follows: 3.506 g of SnCl4.5H2O was weighed,13.280 g TiCl4, 2.73 g FeCl3 6H2O, 1.363 g ZnCl2 (metal mole percent 10%: 70%: 10%:10%), dissolve it in 500 mL of water, stir well and dissolve, add 15% ammonia to the solution to pH 9 and allow to standAfter 15 hours of elution, washed with deionized water three times. The washed solids were placed in an oven at 100 ° C for 20 hours. bakeThe dried solid powder was thoroughly ground and passed through a 200 mesh sieve, and a certain amount of ammonium phosphate was weighed (the mass to solid powder was1: 5), which is thoroughly mixed with the finely ground solid powder. The mixed solid powder was placed in a muffle furnace,500 oC for 5 h to obtain solid acid catalyst.Among them, the metal chloride is tin tetrachloride, titanium tetrachloride, zinc chloride, ferric chloride composed of a mixture. The ammonium salt is phosphorusAmmonium acid.
References:
Shaanxi extended oil (Group) Co., Ltd. Refining and Chemical Company;Li, Jun;Wang, Junfeng;Zhang, Haowen;Tian, Jinguang;Li, Cheng;Cheng, Jingrun;Liu, Junxia;Li, Hongxiong;Zhang, Rong;Li, Jia CN106554277, 2017, A Location in patent:Paragraph 0015; 0016; 0017; 0018; 0019; 0020; 0021-0027
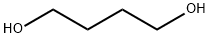
110-63-4
731 suppliers
$24.80/250g
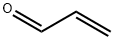
107-02-8
0 suppliers
$38.60/4s8501

1070-70-8
242 suppliers
$5.00/5g
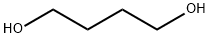
110-63-4
731 suppliers
$24.80/250g
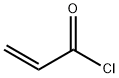
814-68-6
396 suppliers
$21.21/5gm:

1070-70-8
242 suppliers
$5.00/5g
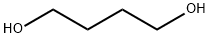
110-63-4
731 suppliers
$24.80/250g
140-10-3
645 suppliers
$5.00/10g
150-76-5
817 suppliers
$5.00/5 g

79-10-7
737 suppliers
$20.16/100 mL

1070-70-8
242 suppliers
$5.00/5g
110-63-4
731 suppliers
$24.80/250g
292638-85-8
5 suppliers
inquiry
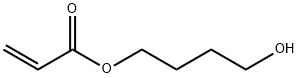
2478-10-6
185 suppliers
$5.00/25g

1070-70-8
242 suppliers
$5.00/5g