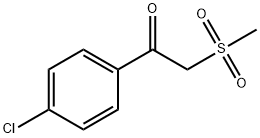
1-(4-CHLOROPHENYL)-2-(METHYLSULFONYL)ETHANONE synthesis
- Product Name:1-(4-CHLOROPHENYL)-2-(METHYLSULFONYL)ETHANONE
- CAS Number:24437-48-7
- Molecular formula:C9H9ClO3S
- Molecular Weight:232.68
![Carbonodithioic acid, S-[2-(4-chlorophenyl)-2-oxoethyl] O-ethyl ester](/CAS/20210305/GIF/91193-23-6.gif)
91193-23-6
1 suppliers
inquiry
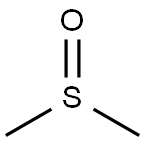
67-68-5
1315 suppliers
$15.00/100g

24437-48-7
27 suppliers
$14.00/250mg
Yield:24437-48-7 86%
Reaction Conditions:
Stage #1: dimethyl sulfoxidewith ferrous(II) sulfate heptahydrate;dihydrogen peroxide at 5 - 10; for 0.5 h;
Stage #2: O-ethyl S-2-(4-chlorophenyl)-2-oxoethyl carbonodithioate at 5 - 20; for 2.5 h;
Steps:
Representative Experimental Procedure for the Synthesis ofCompound 3a
General procedure: 34% H2O2 (2 mL, 20 mmol) was added to a cooled solution ofFeSO4·7H2O (2.78 g, 10 mmol) in DMSO (40 mL), and themixture was stirred for 30 min at 5-10 °C. Then, xanthate 1a(2.4 g, 10 mmol), FeSO4·7H2O (2.78 g, 10 mmol), and H2O2(2 mL,20 mmol) were added and the reaction mixture was stirred for30 min at 5-10 °C and, then, 2 h at r.t. (TLC control). The reactionmixture was poured into H2O (30 mL) and extracted withEtOAc (4 × 50 mL). The combined organic phases were washedwith brine, dried over anhydrous Na2SO4, filtered, and evaporatedunder reduced pressure. The resulting crude mixture ofproducts was purified by silica column chromatography usingEtOAc-PE (1:4) as eluents. The yield of compound 3a was 1.71 g(86%).
References:
Chalikidi, Petrakis N.;Uchuskin, Maxim G.;Trushkov, Igor V.;Abaev, Vladimir T.;Serdyuk, Olga V. [Synlett,2018,vol. 29,# 5,art. no. ST-2017-D0789-L,p. 571 - 575] Location in patent:supporting information
1073-67-2
304 suppliers
$7.00/5g
20277-69-4
275 suppliers
$10.00/1g

24437-48-7
27 suppliers
$14.00/250mg
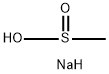
20277-69-4
275 suppliers
$10.00/1g
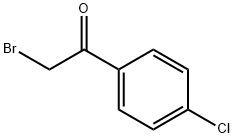
536-38-9
311 suppliers
$9.00/5g

24437-48-7
27 suppliers
$14.00/250mg

1073-67-2
304 suppliers
$7.00/5g

67-68-5
1315 suppliers
$15.00/100g

24437-48-7
27 suppliers
$14.00/250mg
![Benzene, 1-chloro-4-[1-[(trimethylsilyl)oxy]ethenyl]-](/CAS/20200401/GIF/58518-76-6.gif)
