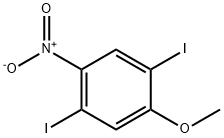
1,4-Diiodo-2-methoxy-5-nitrobenzene synthesis
- Product Name:1,4-Diiodo-2-methoxy-5-nitrobenzene
- CAS Number:55215-55-9
- Molecular formula:C7H5I2NO3
- Molecular Weight:404.93

335349-66-1

55215-55-9
General procedure for the synthesis of 1,4-diiodo-2-methoxy-5-nitrobenzene from 4-iodo-5-methoxy-2-nitroaniline: 4-iodo-5-methoxy-2-nitroaniline (1550 g, 5.27 mmol) was dissolved in a solvent mixture of water and acetonitrile (1:5, v/v) to make a 10 L solution. Subsequently, hydrated p-toluenesulfonic acid (TsOH-H2O, 3615.0 g, 19.0 mmol) was added, and the reaction solution was cooled to 0°C. Under stirring, a 2 L aqueous solution of sodium nitrite (1085.0 g, 15.7 mmol) was added slowly dropwise, and stirring was continued for 1 hr after completion of the dropwise addition. The above solution was slowly dripped into a 2L aqueous solution of potassium iodide (KI, 3061.0 g, 18.4 mmol) and the reaction was carried out for 12 hours at room temperature. After completion of the reaction, the progress of the reaction was monitored by TLC. The reaction solution was poured into water and stirred for 30 min and the solid was collected by filtration. The filter cake was dissolved with ethyl acetate, washed sequentially with saturated sodium thiosulfate solution, saturated sodium bicarbonate solution and saturated brine, dried and concentrated to afford 1,4-diiodo-2-methoxy-5-nitrobenzene (1700 g, 4.2 mmol) in 80% yield.

335349-66-1
9 suppliers
$250.00/1g

55215-55-9
27 suppliers
inquiry
Yield:55215-55-9 80%
Reaction Conditions:
Stage #1: 4-iodo-5-methoxy-2-nitroanilinewith toluene-4-sulfonic acid;sodium nitrite in water;acetonitrile at 0; for 1 h;Milling;Large scale;
Stage #2: with potassium iodide in water;acetonitrile at 20; for 12 h;Milling;Large scale;
Steps:
(3) compound 4 preparation of
The compound 3 (1550g, 5.27 μM) added to the water: b nitrile=1:5 (volume ratio) of 10L solution, then adding a hydrated toluenesulfonates TsOH.H2 O (3615.0g, 19.0 μM), reaction liquid cooling to 0 °C, dropping sodium nitrite (1085.0g, 15.7 μM) aqueous solution of the 2L, after and milling, stirring 1h, then the solution is dropped to KI (3061.0g, 18.4 μM) of the 2L in the aqueous solution, and milling the room temperature after reaction 12h. TLC detection after the reaction, the reaction liquid is poured into the water, stirring 30min, filtering, cake dissolve EA, saturated sodium thiosulfate solution washing, saturated sodium bicarbonate solution washing, saturated salt water washing, drying, turns on lathe does to obtain compound 4 (1700g, 4.2 μM), yield 80%
References:
CN106496037,2017,A Location in patent:Paragraph 0033-0035
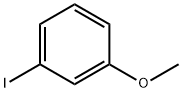
766-85-8
192 suppliers
$22.00/5g

55215-55-9
27 suppliers
inquiry
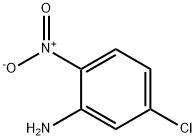
1635-61-6
305 suppliers
$13.00/25g

55215-55-9
27 suppliers
inquiry