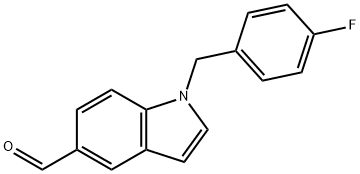
1-(4-fluorobenzyl)-1H-indole-5-carbaldehyde synthesis
- Product Name:1-(4-fluorobenzyl)-1H-indole-5-carbaldehyde
- CAS Number:944893-59-8
- Molecular formula:C16H12FNO
- Molecular Weight:253.27
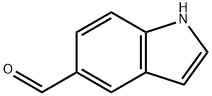
1196-69-6
300 suppliers
$9.00/1g
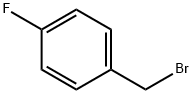
459-46-1
323 suppliers
$10.00/1g

944893-59-8
10 suppliers
$189.00/500mg
Yield:944893-59-8 1.2 g
Reaction Conditions:
with potassium hydroxide in N,N-dimethyl-formamide at 20; for 65 h;Cooling with ice;
Steps:
47 Reference Example 47 1-(4-Fluorobenzyl)-1H-indole-5-carboaldehyde
Reference Example 47 1-(4-Fluorobenzyl)-1H-indole-5-carboaldehyde Indole-5-carboaldehyde (1.0 g) was dissolved in DMF (14 mL), and potassium hydroxide (460 mg) and 4-fluorobenzyl bromide (1.4 g) were sequentially added thereto under ice cooling. The mixture was stirred for 65 hours at room temperature. Water was added to the reaction mixture, and the mixture was extracted with chloroform. The organic layer was washed with saturated brine and then dried over anhydrous sodium sulfate. After distilling off the solvent under reduced pressure, the residue was purified by silica gel column chromatography (20% to 50% ethyl acetate/hexane), and the title compound (1.2 g) was obtained as a pale yellow solid. 1H-NMR (CDCl3) δ: 5.34 (2H, s), 6.72 (1H, dd, J=3.3, 0.9 Hz), 6.98-7.11 (4H, m), 7.21 (1H, d, J=3.3 Hz), 7.36 (1H, d, J=8.6 Hz), 7.76 (1H, dd, J=8.6, 1.6 Hz), 8.18 (1H, d, J=0.9 Hz), 10.03 (1H, s). ESI-MS Found: m/z 254 (M+H)+
References:
US2013/65896,2013,A1 Location in patent:Paragraph 0347-0349