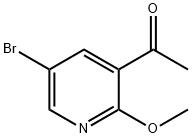
1-(5-BroMo-2-Methoxypyridin-3-yl)ethanone synthesis
- Product Name:1-(5-BroMo-2-Methoxypyridin-3-yl)ethanone
- CAS Number:1256811-02-5
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06
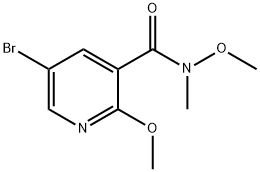
1190128-69-8
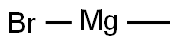
75-16-1

1256811-02-5
Methylmagnesium bromide (MeMgBr, 10 mL, 30.0 mmol) was added slowly and dropwise to a solution of 5-bromo-N,2-dimethoxy-N-methylnicotinamide (5.0 g, 18.18 mmol) in tetrahydrofuran (THF, 50 mL) at -78 °C. The reaction mixture was stirred at 20 °C overnight. Subsequently, the reaction mixture was quenched by pouring into aqueous ammonium chloride (NH4Cl) solution. The mixture was extracted with ethyl acetate (EtOAc). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (eluent: petroleum ether/ethyl acetate=10:1) to afford 1-(5-bromo-2-methoxypyridin-3-yl)ethanone (3.0 g, 71% yield).1H NMR (CDCl3, 400 MHz) δ 8.33 (d, J=2.4Hz, 1H), 8.19 (d, J=2.4Hz, 1H), 4.03 ( s, 3H), 2.63 (s, 3H). Mass spectrum (MS) (M+H)+: 230/232.

1190128-69-8
2 suppliers
inquiry
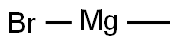
75-16-1
274 suppliers
$12.00/10ml

1256811-02-5
33 suppliers
inquiry
Yield:1256811-02-5 71%
Reaction Conditions:
Stage #1: 5-bromo-N,2-dimethoxy-N-methylnicotinamide;methylmagnesium bromide in tetrahydrofuran at -78 - 20;
Stage #2: with ammonium chloride in tetrahydrofuran;
Steps:
47.3 Step 3 - Synthesis of l-(5-brom -2-methoxypyridin-3-yl)ethanone
To a solution of 5-bromo-N,2-dimethoxy-N-methylnicotinamide (5.0 g, 18.18 mmol) in THF (50 mL), MeMgBr (10 mL, 30.0 mmol) was added dropwise at -78 °C. The reaction mixture was stirred at 20 °C overnight. Then the reaction mixture was added to H4C1 solution. The mixture was extracted with EtOAc. The combined organic phases were washed with brine, dried over Na2S04, filtered and concentrated in vacuo. The crude product was purified using column chromatography (eluted with petroleum ether : EtOAc = 10 : 1) to provide l-(5-bromo-2-methoxypyridin-3-yl)ethanone (3.0 g, yield: 71%). 1H- MR (CDC13, 400 MHz) δ 8.33 (d, J= 2.4 Hz, 1H), 8.19 (d, J= 2.4 Hz, 1H), 4.03 (s, 3H), 2.63 (s, 3H). MS (M+H)+: 230 / 232
References:
WO2013/33971,2013,A1 Location in patent:Page/Page column 145
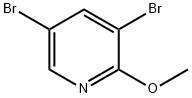
13472-60-1
166 suppliers
$6.00/250mg
3375-31-3
553 suppliers
$23.00/100mg

1256811-02-5
33 suppliers
inquiry

1190128-69-8
2 suppliers
inquiry

1256811-02-5
33 suppliers
inquiry
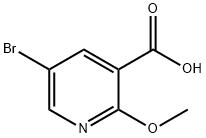
54916-66-4
171 suppliers
$7.00/250mg

1256811-02-5
33 suppliers
inquiry
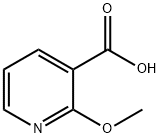
16498-81-0
263 suppliers
$5.00/1g

1256811-02-5
33 suppliers
inquiry