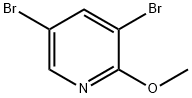
2-METHOXY-3,5-DIBROMO-PYRIDINE synthesis
- Product Name:2-METHOXY-3,5-DIBROMO-PYRIDINE
- CAS Number:13472-60-1
- Molecular formula:C6H5Br2NO
- Molecular Weight:266.92
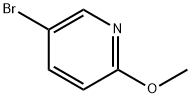
13472-85-0

13472-60-1
The general procedure for the synthesis of 2-methoxy-3,5-dibromopyridine from 5-bromo-2-methoxypyridine was as follows: bromine (17.8 g, 111 mmol) was slowly added to a mixed solution containing 5-bromo-2-methoxypyridine (12.0 g, 63.8 mmol), sodium acetate (5.23 g, 63.8 mmol) and acetic acid (65 mL). The reaction mixture was stirred at 80 °C for 4 hours, followed by continued stirring at room temperature for 12 hours. After completion of the reaction, the mixture was diluted with ether and washed sequentially with water, saturated sodium bicarbonate solution and brine. The organic layer was separated, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography with the eluent being a petroleum ether solution of 1-5% ethyl acetate to give 10.6 g of the target product 2-methoxy-3,5-dibromopyridine. The product was characterized by 1H NMR (300 MHz, DMSO-d6) with chemical shifts of 8.20-8.40 (m, 2H) and 3.92 (s, 3H).

13472-85-0
523 suppliers
$8.00/1g

13472-60-1
166 suppliers
$6.00/250mg
Yield:-
Reaction Conditions:
with bromine;sodium acetate in acetic acid at 20 - 80;
Steps:
10.1
Step 1: To 5-bromo-2-methoxypyridine (0.564 g, 3.0 mmol) and sodium acetate (0.246 g, 3.0 mmol) in acetic acid (3 mL) was added bromine (0.27 mL, 5.25 mmol), and the mixture was stirred at 80° C. for 3 hours, and then at room temperature overnight. The mixture was diluted with H2O and extracted with EtOAc, and the combined organic layers were washed with saturated aqueous Na2CO3 and saturated aqueous Na2S2O3. The organic layer was concentrated and dried under high vacuum to give 3,5-dibromo-2-methoxy-pyridine.
References:
AMIRA PHARMACEUTICALS, INC. US2010/81673, 2010, A1 Location in patent:Page/Page column 14
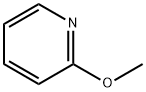
1628-89-3
341 suppliers
$17.96/25g

13472-60-1
166 suppliers
$6.00/250mg
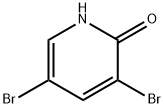
13472-81-6
209 suppliers
$9.00/1g

74-88-4
357 suppliers
$15.00/10g

13472-60-1
166 suppliers
$6.00/250mg
75806-85-8
194 suppliers
$13.00/1g

13472-60-1
166 suppliers
$6.00/250mg

1628-89-3
341 suppliers
$17.96/25g

13472-85-0
523 suppliers
$8.00/1g

13472-60-1
166 suppliers
$6.00/250mg