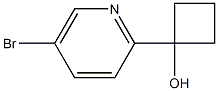
1-(5-BroMopyridin-2-yl)cyclobutanol synthesis
- Product Name:1-(5-BroMopyridin-2-yl)cyclobutanol
- CAS Number:1319256-44-4
- Molecular formula:C9H10BrNO
- Molecular Weight:228.0858
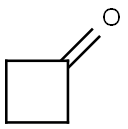
1191-95-3
329 suppliers
$10.00/1g
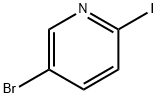
223463-13-6
387 suppliers
$5.00/1g

1319256-44-4
11 suppliers
inquiry
Yield:1319256-44-4 75%
Reaction Conditions:
Stage #1: 5-bromo-2-iodopyridinewith n-butyllithium in hexane;toluene at -78; for 1 h;
Stage #2: cyclobutanone in hexane;toluene at 78; for 2 h;
Steps:
Step 1
To the solution of 5-bromo-2-iodopyridine (5.0 g, 17.67 mmol) in toluene (50 mL) was added n- BuLi (7.07 mL, 17.67 mmol, 2.5 M in n-hexane) at -78 °C; and the mixture was stirred for 1 h at this temperature. Cyclobutanone (1.24 g, 17.67 mmol) and added and the mixture was stirred at -78 °C for further 2 h. The reaction mixture was quenched with saturated aqueous ammonium chloride (50 mL), the organic layer was separated and the aqueous layer was extracted with MTBE (2 x 50 mL). The combined organic layers were washed by brine, dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica (0-15% tert-butyl methyl ether/petroleum ether) to give 1-(5- bromopyridin-2-yl)cyclobutan-1-ol (3.0 g, 75% yield) as a light yellow oil.1H NMR (400 MHz, CDCl3) δ: 8.58 (d, J = 2.2 Hz, 1H), 7.86 (dd, J = 8.4, 2.4 Hz,, 1H), 7.49 (d, J = 8.4 Hz,, 1H), 4.67 (s, 1H), 2.57-2.45 (m, 4H), 2.12-2.04 (m, 1H), 1.92-1.82 (m, 1H).
References:
WO2022/38365,2022,A2 Location in patent:Page/Page column 51