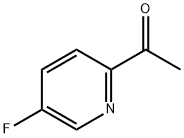
1-(5-Fluoropyridin-2-yl)ethanone synthesis
- Product Name:1-(5-Fluoropyridin-2-yl)ethanone
- CAS Number:915720-54-6
- Molecular formula:C7H6FNO
- Molecular Weight:139.13
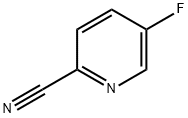
327056-62-2
272 suppliers
$5.00/1g

75-16-1
276 suppliers
$12.00/10ml

915720-54-6
87 suppliers
$36.00/100mg
Yield:915720-54-6 95%
Reaction Conditions:
in tetrahydrofuran;diethyl ether at -78 - 20; for 7.25 h;Inert atmosphere;
Steps:
68.A Step A: 1-(5-Fluoropyridin-2-yl)ethanone
To the solution of 5-fluoropyridine-2-carbonitrile (7.00 g, 57.3 mmol) in tetrahydrofuran (140 mL) cooled to -78 °C under argon atmosphere 3 M methylmagnesium bromidesolution in diethyl ether (22.9 mL, 68.8 mmol) was added dropwise during 15 minutes. Reaction mixture was stirred at -78 °C for 2 hours, and then at room temperature for 5 hours. A solid precipitated from the reaction mixture. To the reaction mixture 2 M hydrochloric acid solution was added until reaching pH = 6 and dissolution of the precipitate. Then to the mixture 6% sodium hydrogencarbonate solution (100 mL) wasadded. The mixture was extracted with dichloromethane (3 x 150 mL). Organic phases were combined, washed twice with water, brine, dried (Na2504) and evaporated under reduced pressure. The residue was purified by column chromatography (silica gel, eluent:dichloromethane 100% to dichloromethane:acetone, 97:3, v/v) to obtain the product as a light yellow oil with a yield of 95% (7.57 g, 54.4 mmol).
References:
WO2015/118434,2015,A1 Location in patent:Page/Page column 72
41404-58-4
407 suppliers
$5.00/1g
97674-02-7
232 suppliers
$15.00/1g

915720-54-6
87 suppliers
$36.00/100mg

41404-58-4
407 suppliers
$5.00/1g

112713-84-5
9 suppliers
inquiry

915720-54-6
87 suppliers
$36.00/100mg

41404-58-4
407 suppliers
$5.00/1g
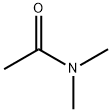
127-19-5
879 suppliers
$5.00/25g

915720-54-6
87 suppliers
$36.00/100mg

41404-58-4
407 suppliers
$5.00/1g
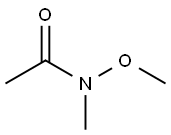
78191-00-1
290 suppliers
$6.00/5g

915720-54-6
87 suppliers
$36.00/100mg