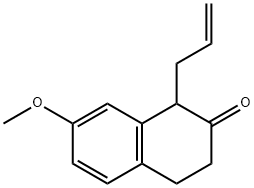
1-Allyl-7-Methoxy-2-tetralone synthesis
- Product Name:1-Allyl-7-Methoxy-2-tetralone
- CAS Number:29093-46-7
- Molecular formula:C14H16O2
- Molecular Weight:216.28
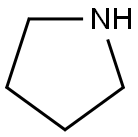
123-75-1
577 suppliers
$10.00/5g
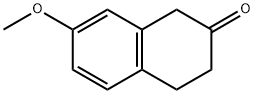
4133-34-0
287 suppliers
$15.00/1g
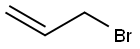
106-95-6
427 suppliers
$10.00/5g

29093-46-7
9 suppliers
inquiry
Yield:-
Reaction Conditions:
in benzene;
Steps:
1 1-Allyl-3,4-dihydro-7-methoxy-2(1H)naphthalenone
EXAMPLE 1 STR48 1-Allyl-3,4-dihydro-7-methoxy-2(1H)naphthalenone To a solution of 104.1 g (0.59 mole) 3,4-dihydro-7-methoxy-2(1H)naphthalenone in 100 ml dry benzene was added dropwise under nitrogen with stirring at room temperature 62.5 g (0.88 mole) pyrrolidine. After the addition (10-15 min), the reaction mixture was refluxed for 2.5 hr with azeotropic removal of water and then cooled to room temperature. The enamine solution was added dropwise to 143.8 g (1.19 mole) allyl bromide with stirring at a rate sufficient to maintain normal refluxing to provide a heavy precipitate. Benzene (50 ml) was added to facilitate stirring and reflux continued for 4 hr; then, 700 ml of water was added and refluxing resumed. After 2 hr, the reaction mixture was cooled to room temperature and diluted with 100 ml benzene. The benzene layer was separated and the aqueous phase extracted with benzene (2*100 ml). The combined extracts were washed with water (2*100 ml) and dried (Na2 SO4). After evaporation of the solvent, the residue was distilled to give 113.5 g (89%) of VIIIa, b.p. 114°-118°/0.1-0.05 mm Hg. NMR: δ 2.40-3.10 (m, 6, allylic and alicyclic), 3.45 (t, 1, C1 --H, J=6.5), 3.75 (s, 3, OCH3), 4.8-6.1 (m, 3, olefinic), 6.7-7.2 (m, 3, ArH). IR: (neat) 1715 cm-1.
References:
US4150032,1979,A