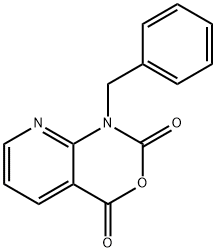
1-benzyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione synthesis
- Product Name:1-benzyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione
- CAS Number:97484-73-6
- Molecular formula:C14H10N2O3
- Molecular Weight:254.24
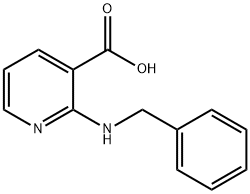
33522-80-4
43 suppliers
$65.00/100mg
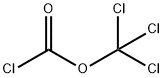
503-38-8
123 suppliers
$15.00/5g
![1-benzyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione](/CAS2/GIF/97484-73-6.gif)
97484-73-6
14 suppliers
$382.00/1g
Yield:97484-73-6 68%
Reaction Conditions:
in 1,4-dioxane; for 8 h;Heating / reflux;
Steps:
2
Trichloromethyl CHLOROFORMATE (2.5 mL, 21 mmol) was added slowly to a suspension of (1) (4 g, 17.5 mmol) in dioxane and refluxed for 8 h under nitrogen atmosphere. The solution was cooled and the solvent was removed under vacuum. The residue was dissolved in dichloromethane and washed by saturated NAHCO3 solution. The organic phase was dried over NA2SO4 and evaporated to yield a residue. The residue was recrystallized by ether to yiels 3.02 g (68 %) of 1-BENZYL, LH-PYRIDO [2,3- D] [1, 3] oxazine-2,4-dione (2) as white solids. MP: 168 °C ; 1H-NMR (DMSO-d6) : d 5.35 (s, 2H), 7.26 (m, 1H), 7.30 (m, 2H), 7.39 (m, 3H), 8.41 (dd, J= 1.5, 7.5 Hz, 1H), 8. 72 (dd, J = 1. 5,7. 5 Hz, 1H) ; EIMS : 277 (M+23).
References:
WO2005/21546,2005,A1 Location in patent:Page/Page column 112; 160

100-44-7
659 suppliers
$13.50/250G
![1H-pyrido[2,3-d][1,3]oxazine-2,4-dione](/CAS2/GIF/21038-63-1.gif)
21038-63-1
32 suppliers
$62.00/100mg
![1-benzyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione](/CAS2/GIF/97484-73-6.gif)
97484-73-6
14 suppliers
$382.00/1g

100-39-0
439 suppliers
$10.00/10g
![1-benzyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione](/CAS2/GIF/97484-73-6.gif)
97484-73-6
14 suppliers
$382.00/1g
5860-70-8
49 suppliers
$60.00/100mg
![1-benzyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione](/CAS2/GIF/97484-73-6.gif)
97484-73-6
14 suppliers
$382.00/1g