
(1-Benzyl-piperidin-4-yl)-isopropyl-aMine synthesis
- Product Name:(1-Benzyl-piperidin-4-yl)-isopropyl-aMine
- CAS Number:132442-32-1
- Molecular formula:C15H24N2
- Molecular Weight:232.36
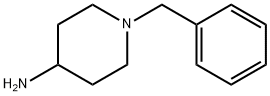
50541-93-0
355 suppliers
$6.00/5g

67-64-1
0 suppliers
$19.10/10ml

132442-32-1
6 suppliers
$370.00/1g
Yield:132442-32-1 96%
Reaction Conditions:
Stage #1: 4-amino-1-benzylpiperidine;acetone in dichloromethane at 20; for 2.5 h;
Stage #2: with sodium tris(acetoxy)borohydride in dichloromethane at 0 - 25; for 2.5 h;
Stage #3: with hydrogenchloride;sodium hydroxideProduct distribution / selectivity;more than 3 stages;
Steps:
3.B.A
Example 3B Preparation of 4-Isopropylamino-l- (4-methoxypyrid-3-ylmethyl) piperidine Monobenzoic Acid Salt Step A-Preparation of l-Benzyl-4-isopropylaminopiperidine To a 50 L 3-neck round-bottom reaction flask equipped with a mechanical stirrer, temperature probe, nitrogen inlet and cooling bath was added 4-amino-1-benzylpiperidine (2,000 g, 10.5 mol) and dichloromethane (20 L). Acetone (610.5 g, 10.5 mol) was added and the reaction mixture was stirred at room temperature for 2.5 hours. The reaction mixture was then cooled to 0 °C to 5 °C with an ice/methanol bath and sodium triacetoxyborohydride (2,673 g, 12.6 mol) was added while maintaining the temperature of the reaction mixture below 25 °C. The cooling bath was then removed and the reaction mixture was stirred until less than 1% starting material was present by GC analysis (about 3 hours). Concentrated hydrochloric acid was added until the pH of the reaction mixture was 7 (about 500 mL). The resulting slurry was filtered through a polypropylene filter pad and the solids were washed with dichloromethane (2 x 2 L). The solids were saved for use after concentration of the filtrate. The filtrate was concentrated at 40 °C until no condensate remained. In a 40 L separatory funnel, the solids and distillation residue were dissolved in water (15 L) and concentrated hydrochloric acid was added until the pH of the solution was 3 (about 2.5 L). The aqueous layer was then washed with dichloromethane (2 x 2 L). The pH of the aqueous layer was adjusted to 11 to 12 with 50% aqueous sodium hydroxide solution (about 4.5 L) and this mixture was extracted with dichloromethane (5 x 3L). The organic layers were combined, decolorized with charcoal (50 g) and dried over anhydrous magnesium sulfate (200 g). The solids were filtered off using a glass fiber filter pad and the filtrate was concentrated until no condensate remained to afford the title compound (2,336 g, 96% yield).
References:
WO2005/42514,2005,A2 Location in patent:Page/Page column 29
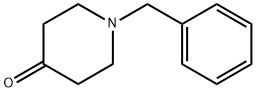
3612-20-2
448 suppliers
$6.00/10g
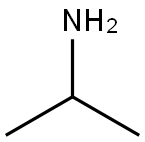
75-31-0
443 suppliers
$14.00/25mL

132442-32-1
6 suppliers
$370.00/1g