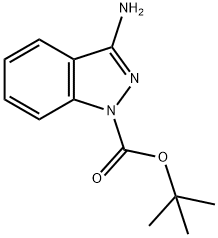
1-Boc-3-aminoindazole synthesis
- Product Name:1-Boc-3-aminoindazole
- CAS Number:1204298-58-7
- Molecular formula:C12H15N3O2
- Molecular Weight:233.27

24424-99-5
811 suppliers
$13.50/25G
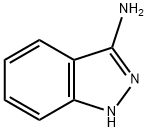
874-05-5
201 suppliers
$15.00/1g

1204298-58-7
38 suppliers
$45.00/10mg
Yield:1204298-58-7 48%
Reaction Conditions:
with dmap;triethylamine in tetrahydrofuran at 20; for 1.5 h;
Steps:
28A Example 28A tert-l3utyl 3-amino-i H-indazole-1 -carboxylate
150mg (1.13 mmol) of 1 H-indazole-3-amine were initially charged in 3 ml of THF, 320 mg (1.46 mmol) of di-tert-butyl dicarbonate, 137 mg (1.35 mmol) of triethylamine and 48 mg (0.39 mmol) of dimethylaminopyridine were then added and the mixture was stirred at RT for 1.5 h. The reaction solution was diluted with ethyl acetate and washed in each case once with water, saturated aqueous ammonium chloride solution and saturated sodium chloride solution. The organic phase was dried over sodium sulphate and filtered and the filtrate was concentrated. The residue was purified by silica gel chromatography (mobile phase:cyclohexane/ethyl acetate 3/i->i/i). This gave 126 mg of the target compound (48% of theory).10457] LC-MS (Method 2): R=0.88 mm10458] MS (ESpos): mlz=234 (M+H)10459] ‘H-NMR (400 MHz, DMSO-d5): ?=i.58 (s, 9H),6.30 (s, 2H), 7.25 (t, 1H), 7.50 (t, 1H), 7.82 (d, 1H), 7.94 (d, 1H).
References:
US2016/362408,2016,A1 Location in patent:Paragraph 0455; 0456; 0457; 0458; 0459
870-46-2
523 suppliers
$5.00/10g
4387-36-4
241 suppliers
$9.00/1g

1204298-58-7
38 suppliers
$45.00/10mg

870-46-2
523 suppliers
$5.00/10g
2042-37-7
348 suppliers
$7.00/10g

1204298-58-7
38 suppliers
$45.00/10mg