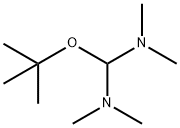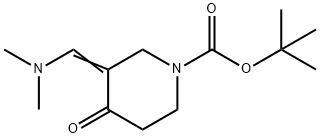
1-Boc-3-[(Dimethylamino)methylene]-4-oxopiperidine synthesis
- Product Name:1-Boc-3-[(Dimethylamino)methylene]-4-oxopiperidine
- CAS Number:157327-41-8
- Molecular formula:C13H22N2O3
- Molecular Weight:254.33

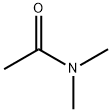
79099-07-3
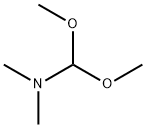
4637-24-5
![1-Boc-3-[(Dimethylamino)methylene]-4-oxopiperidine](/CAS/GIF/157327-41-8.gif)
157327-41-8
The general procedure for the synthesis of 1-Boc-3-[(dimethylamino)methylene]-4-oxopiperidine from N-tert-butoxycarbonyl-4-piperidone and N,N-dimethylformamide dimethyl acetal was as follows: N,N-dimethylformamide dimethyl acetal (18 g, 20 mL, 151 mmol) was mixed with 1-(tert-butoxycarbonyl)piperidin-4-one (30 g, 151 mmol) were dissolved in N,N-dimethylformamide (240 mL) and the reaction was stirred at 80°C for 24 hours. After completion of the reaction, the reaction mixture was concentrated under reduced pressure to afford the target product 1-Boc-3-[(dimethylamino)methylene]-4-oxopiperidine as a yellow solid (38.4 g, 100% yield). Mass spectrum (ESI, positive ion mode) m/z: 255 [M + H]+.

79099-07-3
0 suppliers
$5.00/5g

4637-24-5
646 suppliers
$5.00/25g
![1-Boc-3-[(Dimethylamino)methylene]-4-oxopiperidine](/CAS/GIF/157327-41-8.gif)
157327-41-8
64 suppliers
$24.00/1g
Yield:157327-41-8 100%
Reaction Conditions:
in N,N-dimethyl-formamide at 80; for 24 h;
Steps:
24.1 Step 1) 1-(tert-butoxycarbonyl)-3-((dimethylamino)methylene)piperidin-4-one
A mixture of N,N-dimethylformamide dimethyl acetal (18 g, 20 mL, 151 mmol) and l-(tert-butoxycarbonyl)piperidin-4-one (30 g, 151 mmol) in DMF (240 mL) was stirred at 80 °C for 24 h. The mixture was concentrated in vacuo to give the title compound as a yellow solid (38.4 g, 100%). MS (ESI, pos. ion) m/z: 255 [M + H]+.
References:
WO2014/89324,2014,A1 Location in patent:Paragraph 0308

79099-07-3
0 suppliers
$5.00/5g
1188-33-6
273 suppliers
$11.19/5ML
![1-Boc-3-[(Dimethylamino)methylene]-4-oxopiperidine](/CAS/GIF/157327-41-8.gif)
157327-41-8
64 suppliers
$24.00/1g