
1-BOC-4-CYANO-4-(2-CHLOROPHENYL)-PIPERIDINE synthesis
- Product Name:1-BOC-4-CYANO-4-(2-CHLOROPHENYL)-PIPERIDINE
- CAS Number:186347-31-9
- Molecular formula:C17H21ClN2O2
- Molecular Weight:320.81
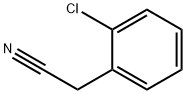
2856-63-5
338 suppliers
$17.00/10g
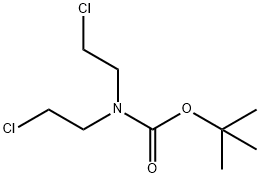
118753-70-1
177 suppliers
$6.00/1g

186347-31-9
13 suppliers
inquiry
Yield:186347-31-9 86%
Reaction Conditions:
Stage #1: 2-Chlorophenylacetonitrilewith sodium hydride in DMF (N,N-dimethyl-formamide);oil at 0; for 0.833333 h;
Stage #2: N-(tert-butyloxycarbonyl)bis(2-chloroethyl)amine in DMF (N,N-dimethyl-formamide);oil at 0 - 75; for 5.75 h;
Steps:
1 4-(2-Chlorophenyl)-4-cyanopiperidine-1-carboxylic acid tert-butyl ester (Boc-29.237)
To a suspension of NaH (60% oil suspension, 7.37 g, 184.4 mmol) in DMF (110 mL), cooled by ice/water, was added a solution of the nitrile 35.237 (9.782 g, 64.52 mmol) in DMF (20 mL) over 20 min. Hydrogen evolved, and the reaction mixture turned yellow. After 30 min, a solution of the chloroamine 34 (14.88 g, 61.45 mmol) in DMF (15 mL) was added. After 15 min the cooling bath was removed, and the reaction mixture was heated to 75 C. (bath temperature) for 5.5 h. TLC indicated the complete consumption of both starting materials. The DMF was evaporated. Upon addition of water and ether (200 mL each) a solid separated, which was filtered off, washed thoroughly with ether and EtOAc, and dried, yielding 8.018 g (24.99 mmol, 41%) of Boc-29.237. The layers of the combined filtrte and washings were separated, and the aqueous layer was extracted with more ether (2x150 mL). The combined organic layers were washed with 5% HOAc, water, IN NaOH, water, and brine and dried over MgSO4. This solution was ltered through a pad of silica gel. Upon concentration a solid precipitated, which was ltered off, washed with ether and hexanes and dried, yielding 6.138 g (19.13 mmol, 31%) of Boc-29.237. The mother liquor was concentrated and the residue purified by column chroma-tography on silica gel, yielding 2.885 g (8.99 mmol, 15%) of Boc-29.237. The total yield was 17.04 g (53.12 mmol, 86%), mp 165-166 C. 2H NMR (CDC13, 200 MHz): 8=1.48 (s, 9H), 2.00 (brdt, J=4.2,13.0 Hz, 2H), 2.43-2.53 (m, 2H), 3.28 (brt, J=12.8 Hz, 2H), 4.28 (brd, J=13.2 Hz, 2H), 7.28-7.50 (m, 4H). C17H21C1N2O2 (320.82): calcd. C 63.65, H 6.60, Cl 11.05, N 8.73; found C 63.82, H 6.61, Cl 10.90, N 8.72.
References:
US2003/229067,2003,A1 Location in patent:Page 26-27

24424-99-5
871 suppliers
$13.50/25G

186347-31-9
13 suppliers
inquiry