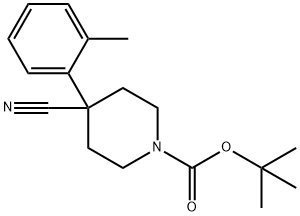
1-BOC-4-CYANO-4-(2-METHYLPHENYL)-PIPERIDINE synthesis
- Product Name:1-BOC-4-CYANO-4-(2-METHYLPHENYL)-PIPERIDINE
- CAS Number:186347-28-4
- Molecular formula:C18H24N2O2
- Molecular Weight:300.4
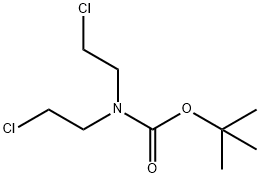
118753-70-1
178 suppliers
$6.00/1g
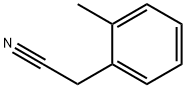
22364-68-7
270 suppliers
$6.00/10g

186347-28-4
6 suppliers
inquiry
Yield:186347-28-4 33%
Reaction Conditions:
with sodium hydride in Pionier 2076;N,N-dimethyl-formamide at 20 - 70; for 6 h;Inert atmosphere;
Steps:
18.A
Part A: Sodium hydride (60% in mineral oil, 8.51 g, 212 mmol) was added in portions to 67 (10.0g, 76.2 mmol) and i-butyl bis(2-chloroethyl)carbamate (20.3 g, 83.8 mmol) in DMF (150 mL) over a 3 h period under nitrogen. After the addition was complete the reaction was stirred for 1 h at room temperature then 2 h at 70 °C. The reaction was cooled to room temperature and the volume was reduced to -60 mL in vacuo. Saturated ammonium chloride (100 mL) was added with vigorous stirring. The aqueous layer was decanted and the oily solid residue taken up in methylene chloride. The reaction was repeated and combined for purification by chromatography on silica gel (methylene chloride/ethyl acetate) to provide 68 (15.0 g, 33% yield) as a light brown solid: 1H NMR (400 MHz, CDCI3) 7.27-7.22 (m, 4H), 4.42-4.18 (m, 2H), 3.37-3.20 (m, 2H), 2.65 (s, 3H), 2.36-2.28 (m, 2H), 1.90 (td, J = 9.8, 3.1 Hz, 2H), 1.48 (s, 9H).
References:
WO2011/46771,2011,A1 Location in patent:Page/Page column 156

24424-99-5
819 suppliers
$13.50/25G

186347-28-4
6 suppliers
inquiry