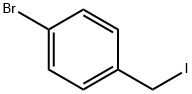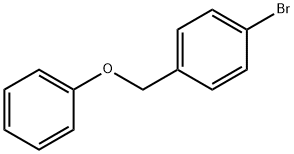
1-BROMO-4-(PHENOXYMETHYL)BENZENE synthesis
- Product Name:1-BROMO-4-(PHENOXYMETHYL)BENZENE
- CAS Number:20600-22-0
- Molecular formula:C13H11BrO
- Molecular Weight:263.13
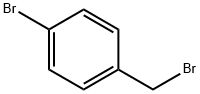
589-15-1
437 suppliers
$6.00/10g

108-95-2
786 suppliers
$14.00/25g

20600-22-0
18 suppliers
$251.22/5g
Yield:20600-22-0 93%
Reaction Conditions:
Stage #1: phenolwith N,N,N-trimethyl-2-hydroxyethyl-ammonium hydroxide in lithium hydroxide monohydrate at 20; for 0.25 h;
Stage #2: 1-bromo-4-(bromomethyl)benzene in lithium hydroxide monohydrate at 20; for 24 h;
Steps:
the synthesis of O-substituted phenolic derivatives.[13]
General procedure: A typical procedure: A flask was charged with phenol (3.0 mmol) and choline hydroxide (0.66 mL). Then, the mixture was stirred at room temperature in open air for 15 min. Next, benzyl bromide (3.0 mmol) was added into the flask, then the resulting mixture (two phases: top ChOH layer and bottom benzyl bromide layer) allowed to stir at room temperature for 24 h. The reaction mixture (two phases: top product layer and bottom aqueous ChOH layer) was extracted with diethyl ether (3 × 10 mL). The combined organic layers were washed with water, then dried with anhydrous Na2SO4, and evaporated under reduced pressure. The crude mixture was purified by column chromatography on silica gel (hexanes/ethyl acetate). Benzyl phenyl ether (3a); 1H NMR (400 MHz, CDCl3) δ (ppm): 7.48-7.40 (m, 4H), 7.37-7.28 (m, 3H), 7.03-6.97 (m, 3H), 5.10 (s, 2H); 13C NMR (100 MHz, CDCl3) δ (ppm): 158.8, 137.1, 129.5, 128.6, 127.9, 127.5, 120.9, 114.9, 69.9.
References:
Joo, Seong-Ryu;Kim, Seung-Hoi;Kwon, Gyu-Tae;Park, Soo-Youl [Bulletin of the Korean Chemical Society,2020,vol. 41,# 12,p. 1200 - 1205]
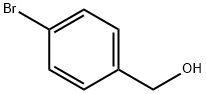
873-75-6
318 suppliers
$6.00/5g
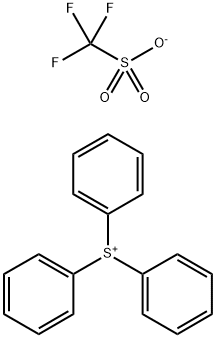
66003-78-9
94 suppliers
$44.00/250mg

20600-22-0
18 suppliers
$251.22/5g
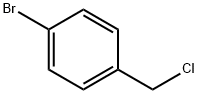
589-17-3
164 suppliers
$10.00/5g

108-95-2
786 suppliers
$14.00/25g

20600-22-0
18 suppliers
$251.22/5g