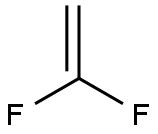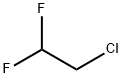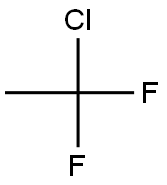
1-Chloro-1,1-difluoroethane synthesis
- Product Name:1-Chloro-1,1-difluoroethane
- CAS Number:75-68-3
- Molecular formula:C2H3ClF2
- Molecular Weight:100.5
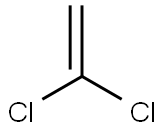
75-35-4
194 suppliers
$20.00/5 g
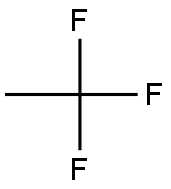
420-46-2
0 suppliers
inquiry

75-68-3
0 suppliers
$35.00/5 g
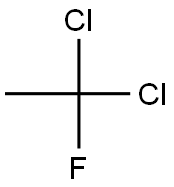
1717-00-6
0 suppliers
$55.00/5 g
Yield:420-46-2 80%
Reaction Conditions:
with hydrogen fluoride;SbCl5 on Carbon at 100; under 760.051 Torr; for 0.00277778 h;
Steps:
3
The vapor phase fluorination reaction is conducted in the same 2.54 cm diameter×81 cm long Monel reactor preceded by a vaporizer as in Examples 1 and 2. The reactor is heated with an electric furnace. The reactor is loaded with 100 ml of SbCl5 supported catalyst that is prepared and activated by the same procedures as described in Example 1. The reactor is kept at 100° C. and at atmospheric pressure. The HF flow rate is adjusted to 0.35 g/min and the 1,1-dichloroethylene (VDC) is started at 0.28 g/min. This provides a mole ratio of HF to VDC of about 8:1. The contact time is about 10 seconds. The reaction is run at these conditions until stable reaction conditions are reached. The reaction is monitored by taking reactor effluent samples directly into an in-line GC as described in Examples 1 and 2. The conversion of the VDC is >95% on a molar basis. The desired HFC-143a product is produced in good yields (>80% on a molar basis) at these conditions with the majority of the other products being intermediates HCFC-141b and HCFC-142b.
References:
Honeywell International Inc. US2005/222472, 2005, A1 Location in patent:Page/Page column 3
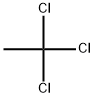
71-55-6
0 suppliers
$24.10/48614

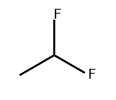
75-68-3
0 suppliers
$35.00/5 g
75-37-6
0 suppliers
$25.00/25 g

75-68-3
0 suppliers
$35.00/5 g

75-35-4
194 suppliers
$20.00/5 g

75-68-3
0 suppliers
$35.00/5 g