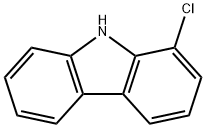
1-Chloro-9H-carbazole synthesis
- Product Name:1-Chloro-9H-carbazole
- CAS Number:5599-70-2
- Molecular formula:C12H8ClN
- Molecular Weight:201.65
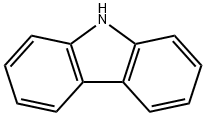
86-74-8

5599-70-2
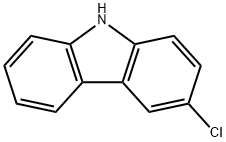
2732-25-4
General procedure for the synthesis of 1-chloro-9H-carbazole and 3-chloro-9H-carbazole from carbazole: Carbazole (0.2 mmol), N-chlorosuccinimide (NCS, 0.26 mmol), camphorsulfonate (D-CSA, 0.1 mmol), and 1,3-bis(1-adamantyl)imidazolium tetrafluoroborate (0.01 mmol) were prepared in a 1,4-dioxane ( 1 mL) was stirred well. The reaction was carried out at room temperature (25 °C) for 24 h under air atmosphere. The reaction progress was monitored by gas chromatography-mass spectrometry (GC-MS). When the carbazole was completely consumed, the reaction was quenched with saturated aqueous sodium bicarbonate (NaHCO3) solution (4 mL). The reaction mixture was extracted with ethyl acetate (EtOAc, 4 mL × 3). The organic layer was washed with pure water (4 mL × 3). The organic layers were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to remove the solvent to give the crude product. Finally, the crude product was purified by silica gel fast column chromatography.
Yield:2732-25-4 87.7% ,5599-70-2 8.3%
Reaction Conditions:
with 1,3-di(adamantan-1-yl)-1H-imidazol-3-ium tetrafluoroborate;N-chloro-succinimide;camphor-10-sulfonic acid in 1,4-dioxane at 25; for 24 h;
Steps:
General Procedure A:
General procedure: A mixture of Acetylaniline (0.2 mmol), NCS (0.26 mmol), D-CSA (0.1 mmol) and 1,3-di(1-adamantl)imidazolium tetrafluoroborate (0.01 mmol) in dioxane (1 mL) wasstirred at room temperature (25 oC) under air atmosphere for 24h. The reactionmonitored by GC-MS. When the acetylaniline was consumed completely, the reactionmixture was quenched with saturated aq. NaHCO3 (4 mL). The resulting mixture wasextracted with EtOAc (4 mL x 3). The organic layer was washed with pure water (4ml x 3). The combined organic layer was dried over anhydrous Na2SO4, filtered andthe solvent was removed under reduced pressure to provide the crude product. Thepurification was performed by flash column chromatography on silica gel.
References:
Chen, Jie;Xiong, Xiaoyu;Chen, Zenghua;Huang, Jianhui [Synlett,2015,vol. 26,# 20,art. no. ST-2015-S0670-C,p. 2831 - 2834] Location in patent:supporting information
53475-34-6
6 suppliers
inquiry

5599-70-2
57 suppliers
$119.00/100mg
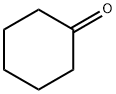
108-94-1
583 suppliers
$12.00/50g

5599-70-2
57 suppliers
$119.00/100mg
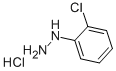
41052-75-9
263 suppliers
$5.00/5g

5599-70-2
57 suppliers
$119.00/100mg

86-74-8
347 suppliers
$10.00/5g
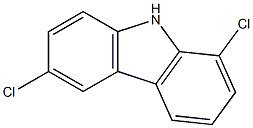
64389-47-5
0 suppliers
inquiry

5599-70-2
57 suppliers
$119.00/100mg
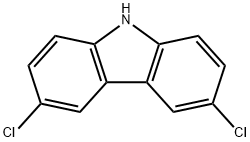
5599-71-3
106 suppliers
$49.00/1g