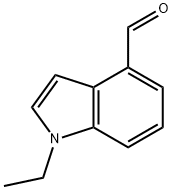
1-ethyl-1H-indole-4-carbaldehyde synthesis
- Product Name:1-ethyl-1H-indole-4-carbaldehyde
- CAS Number:894852-86-9
- Molecular formula:C11H11NO
- Molecular Weight:173.21
1074-86-8

75-03-6

894852-86-9
a) Synthesis of 1-ethyl-1H-indole-4-carboxaldehyde: Sodium hydride (827 mg, 60% dispersion in oil, 20.7 mmol) was added batchwise to an anhydrous N,N-dimethylformamide (DMF, 6.5 mL) solution of 1H-indole-4-carboxaldehyde (2.00 g, 13.8 mmol) under argon protection. The reaction mixture was stirred at room temperature for 30 min. Subsequently, ethidium iodide (2.22 mL, 27.5 mmol) was slowly added dropwise and stirring was continued for 12 hours at room temperature. Upon completion of the reaction, the reaction was quenched by the addition of water (100 mL) and extracted with ethyl acetate (3 x 100 mL). The organic phases were combined, washed with saturated saline (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give an orange oily crude product. Purification by silica gel column chromatography (elution gradient: dichloromethane to 5% methanol/dichloromethane) afforded the target compound 1-ethyl-1H-indole-4-carbaldehyde (2.43 g, 99% yield) as a yellow oil. Its nuclear magnetic resonance hydrogen spectrum (1H NMR, 300 MHz, DMSO-d6) data were as follows: δ 10.19 (s, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.68-7.64 (m, 2H), 7.37-7.32 (m, 1H), 7.08 (d, J = 3.0 Hz, 1H), 4.28 (q, J = 7.2 Hz, 2H), 1.36 (t, J = 7.2 Hz, 3H).

1074-86-8
298 suppliers
$10.00/1g

75-03-6
369 suppliers
$11.00/5g

894852-86-9
29 suppliers
$45.00/50mg
Yield:894852-86-9 99%
Reaction Conditions:
Stage #1: 4-formyl indolewith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: ethyl iodide in N,N-dimethyl-formamide at 20; for 12 h;
Steps:
75.a
a) l-ethyl-lH-indole-4-carbaldehvde;
References:
WO2007/53131,2007,A2 Location in patent:Page/Page column 179-180