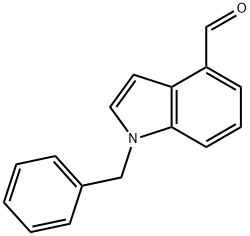
1-(PHENYLMETHYL)-1H-INDOLE-4-CARBOXALDEHYDE synthesis
- Product Name:1-(PHENYLMETHYL)-1H-INDOLE-4-CARBOXALDEHYDE
- CAS Number:192993-85-4
- Molecular formula:C16H13NO
- Molecular Weight:235.28
Yield:-
Reaction Conditions:
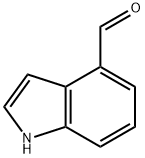
with ammonium chloride in N-methyl-acetamide;
Steps:
1 Synthesis of 1-benzylindole-4-carbaldehyde STR8
EXAMPLE 1 Synthesis of 1-benzylindole-4-carbaldehyde STR8 Sodium hydride (95%; 0.37 g) was suspended in 10 ml of dimethylformamide and cooled to a temperature ranging from 0° to 5° C. with stirring, under an argon gas atmosphere. To the resulting mixed liquid, there was dropwise added a solution of 2.00 g of indole-4-carbaldehyde in 5 ml of dimethylformamide over 15 minutes. After completion of the dropwise addition, the mixture was stirred at a temperature ranging from 0° to 5° C. for 30 minutes. To the reaction solution, there was added a solution of 2.47 g of benzyl bromide in 5 ml of dimethylformamide, followed by stirring at room temperature for 2 hours. The reaction solution was poured into 200 ml of a 10% ammonium chloride aqueous solution, followed by extraction with ethyl acetate (200 ml*2). After washing the extract with a saturated common salt solution, it was dried over anhydrous sodium sulfate, followed by evaporation of the solvent under reduced pressure to give 3.18 g of 1-benzylindole-4-carbaldehyde as brown crystals. The yield thereof was found to be 98%. NMR (CDC13) δ: 5.35 (2H,s), 7.0~7.1 (2H,m), 7.2~7.4 (6H,m), 7.51 (1H,d,J=8.0 Hz), 7.60 (1H,d,J=8.0 Hz), 10.21 (1H,s)
References:
US5811439,1998,A