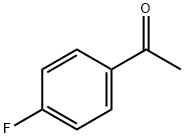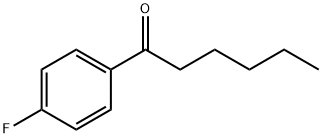
1-Hexanone, 1-(4-fluorophenyl)- synthesis
- Product Name:1-Hexanone, 1-(4-fluorophenyl)-
- CAS Number:1426-70-6
- Molecular formula:C12H15FO
- Molecular Weight:194.25
Yield:1426-70-6 91%
Reaction Conditions:
with Cp*Ir(6,6'-dionato-2,2'-bipyridine)(H2O);Cs2CO3 in tert-Amyl alcohol at 125; for 12 h;Schlenk technique;
Steps:
4.2. General procedure for the cross-coupling of secondary alcoholswith alkenyl primary alcohols catalyzed by [Cp*Ir(2,20-bpyO)(H2O)](cat. 10) (Tables 1-3).
General procedure: To an oven-dried 25 mL Schlenk tube were added secondaryalcohol (1 mmol), alkenyl alcohol (1.2 mmol), catal (0.01 mmol,1 mol%), Cs2CO3 (98 mg, 0.3 mmol, 0.3 equiv) and tert-amyl alcohol(1 mL). The mixture of reaction was heated under 125 C in an oilbath for 12 h. The reaction mixture was cooled to ambient temperature,concentrated in vacuo and purified by flash column chromatographywith hexanes/ethyl acetate to afford thecorresponding product.1-phenylhexan-1-one (3aa) [16b]. Purified by flash columnchromatography on silica gel (ethyl acetate/hexanes = 1/100);88% yield (155 mg)
References:
Xu, Xiangchao;Li, Shun;Luo, Shiyuan;Yang, Jiazhi;Li, Feng [Journal of Catalysis,2022,vol. 413,p. 365 - 373]
850132-22-8
0 suppliers
inquiry

1426-70-6
17 suppliers
inquiry