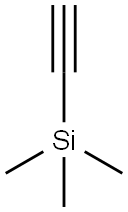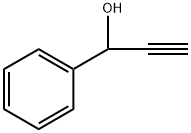
1-PHENYL-2-PROPYN-1-OL synthesis
- Product Name:1-PHENYL-2-PROPYN-1-OL
- CAS Number:4187-87-5
- Molecular formula:C9H8O
- Molecular Weight:132.16

100-52-7

74-86-2

4187-87-5
The general procedure for the synthesis of (±)-1-phenyl-2-propyn-1-ol from benzaldehyde and acetylene was as follows: magnesium (518 mg, 28.3 mmol) was placed in a pre-flame-dried, two-necked, round-bottomed flask fitted with a reflux condenser and cooled to room temperature under argon atmosphere. Subsequently, tetrahydrofuran (30 mL) and a small amount of iodine crystals were added. Next, a portion of the total volume of n-butyl chloride (2.23 mL, 28.3 mmol) was added dropwise and the mixture was refluxed until the Grignard reagent was generated. After removing the unreacted n-butyl chloride, the remaining n-butyl chloride was added. Stirring was continued at room temperature until all the magnesium was consumed. The reaction mixture was cooled to 0°C and acetylene gas was passed into it over a period of 15 minutes. Subsequently, benzaldehyde (1.0 g, 9.4 mmol) dissolved in tetrahydrofuran (20 mL) was added at 0°C and stirred for 6 hours. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride solution, diluted with water and extracted with ethyl acetate. The organic layers were combined, dried with anhydrous sodium sulfate, concentrated and purified by silica gel column chromatography (eluent 10% petroleum ether solution of ethyl acetate) to afford the target product (±)-1-phenyl-2-propyn-1-ol (1.08 g, 86% yield) as a pale yellow oil.

100-52-7
973 suppliers
$17.30/2g
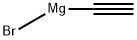
4301-14-8
126 suppliers
$55.20/100ml

4187-87-5
170 suppliers
$9.00/1g
Yield:4187-87-5 100%
Reaction Conditions:
in tetrahydrofuran at 0 - 20; for 20 h;Inert atmosphere;Schlenk technique;
Steps:
1-phenylprop-2-yn-1-ol 7
A solution of benzaldehyde (2.0 mL, 19.7 mmol) in distilled THF (10 mL) was treated at 0°C with ethynylmagnesium bromide (51 mL, 59.1 mmol). The resulting mixture was stirredat 0 °C for 1 h and for 19 h at r.t.. After treatment with a saturated aqueous solution of NH4Cl and extractionswith Et2O, the combined organic layers were washed with brine, dried over MgSO4 and concentrated underreduced pressure. Purification by silica gel chromatography (pentane/EtOAc: 95/5) afforded the expectedpropargyl alcohol 7 as a pale yellow powder (2.60 g, 19.7 mmol, quantitative yield).
References:
Cocq, Kévin;Saffon-Merceron, Nathalie;Poater, Albert;Maraval, Valérie;Chauvin, Remi [Synlett,2016,vol. 27,# 14,art. no. ST-2016-W0336-C,p. 2105 - 2112] Location in patent:supporting information
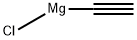
65032-27-1
91 suppliers
$47.60/100ml

100-52-7
973 suppliers
$17.30/2g

4187-87-5
170 suppliers
$9.00/1g

624-67-9
62 suppliers
$120.00/500mg

108-86-1
523 suppliers
$10.00/5g

4187-87-5
170 suppliers
$9.00/1g